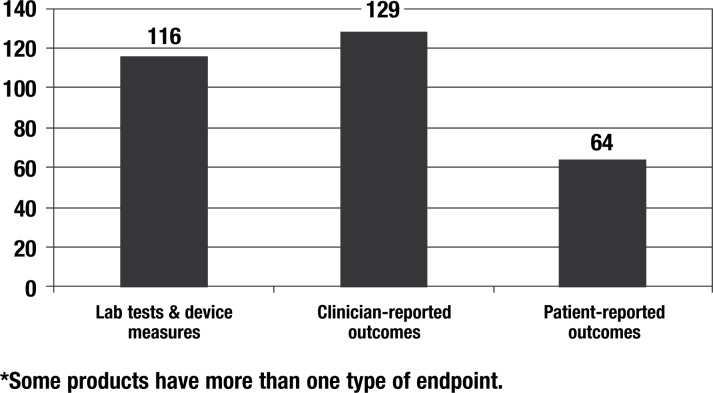
Figure 2. Types of Endpoints in U.S. Product Labels for 215 New Drugs Approved 1997–2002*.
30% of new product labels included PRO endpoints
Reprinted from Willke R, Burke BL, Erickson P. Measuring treatment impact: a review of patient-reported outcomes and other efficacy endpoints in approved labels. Control Clin Trials. 2004;25(6):535–552, with permission from Elsevier.