Abstract
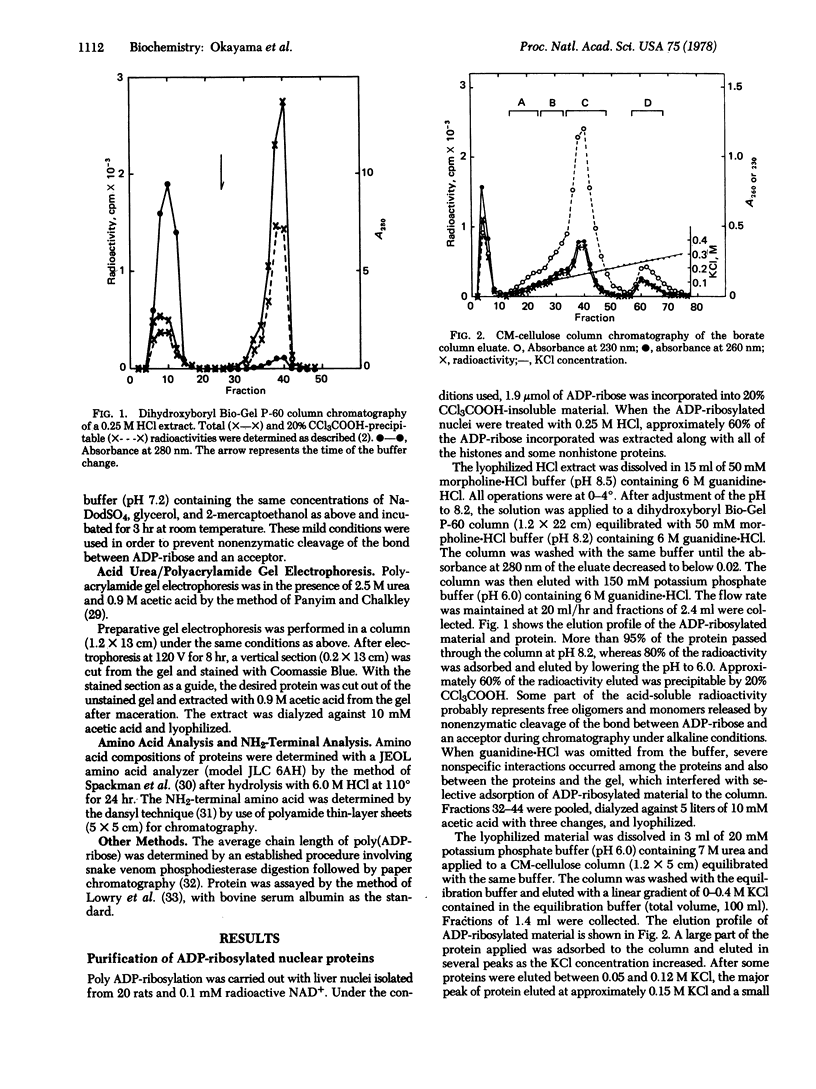
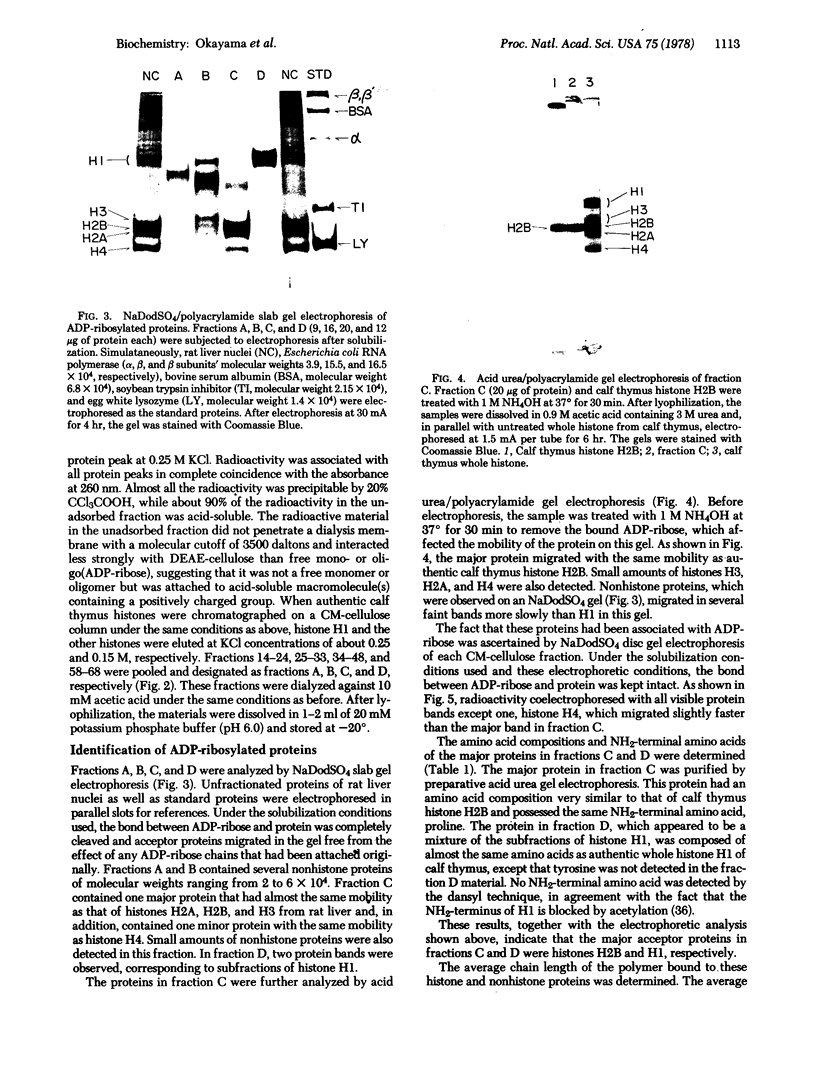
Nuclear proteins modified by mono or poly ADP-ribosylation were selectively isolated and purified by covalent chromatography on a dihydroxyboryl polyacrylamide bead column that specifically interacts with cis-diol-containing compounds. From rat liver nuclei that had been incubated with NAD+, histones and some nonhistone proteins were extracted with 0.25 M HCl. Approximately 60% of the ADP-ribose incorporated into 20% trichloroacetic acid-precipitable material was recovered in this extract. The ADP-ribosylated material was then isolated from the extract by covalent chromatography on a borate gel column and further purified by carboxymethylcellulose column chromatography. As judged by electrophoretic mobilities in various gel systems and by amino acid compositions, approximately 50% of the ADP-ribose recovered in the carboxymethylcellulose fractions was associated with several nonhistone proteins with molecular weights of 2-6 x 10(4), while 35% aand 15% were associated with histones H2B and H1, respectively. Since the average chain length of the polymer bound to any of these proteins was less than two ADP-ribos-l units, the percentage distribution reflects the number of ADP-ribosylated sites rather than the chain length.
Full text
PDF

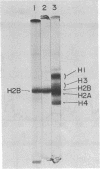
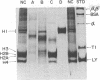
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballal N. R., Goldberg D. A., Busch H. Dissociation and reconstitution of chromatin without appreciable degradation of the proteins. Biochem Biophys Res Commun. 1975 Feb 17;62(4):972–982. doi: 10.1016/0006-291x(75)90418-0. [DOI] [PubMed] [Google Scholar]
- Burzio L., Koide S. S. Mode of inhibition of DNA synthesis induced by adenosine diphosphoribosylation of nuclear protein. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1185–1190. doi: 10.1016/0006-291x(71)90031-3. [DOI] [PubMed] [Google Scholar]
- CHAUVEAU J., MOULE Y., ROUILLER C. Isolation of pure and unaltered liver nuclei morphology and biochemical composition. Exp Cell Res. 1956 Aug;11(2):317–321. doi: 10.1016/0014-4827(56)90107-0. [DOI] [PubMed] [Google Scholar]
- Caplan A. I., Rosenberg M. J. Interrelationship between poly (ADP-Rib) synthesis, intracellular NAD levels, and muscle or cartilage differentiation from mesodermal cells of embryonic chick limb. Proc Natl Acad Sci U S A. 1975 May;72(5):1852–1857. doi: 10.1073/pnas.72.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich L. S., Jaus H., Siebert G. In vivo occurence of bound ADP-ribose. FEBS Lett. 1973 Dec 1;37(2):228–230. doi: 10.1016/0014-5793(73)80465-x. [DOI] [PubMed] [Google Scholar]
- Fujimura S., Hasegawa S., Shimizu Y., Sugimura T. Polymerization of the adenosine 5'-diphosphate-ribose moiety of nicotinamide-adenine dinucleotide by nuclear enzyme. I. Enzymatic reactions. Biochim Biophys Acta. 1967 Sep 26;145(2):247–259. doi: 10.1016/0005-2787(67)90043-3. [DOI] [PubMed] [Google Scholar]
- Hayaishi O., Ueda K. Poly(ADP-ribose) and ADP-ribosylation of proteins. Annu Rev Biochem. 1977;46:95–116. doi: 10.1146/annurev.bi.46.070177.000523. [DOI] [PubMed] [Google Scholar]
- Hilz H., Stone P. Poly(ADP-ribose) and ADP-ribosylation of proteins. Rev Physiol Biochem Pharmacol. 1976;76:1-58, 177. doi: 10.1007/BFb0027686. [DOI] [PubMed] [Google Scholar]
- Inman J. K., Dintzis H. M. The derivatization of cross-linked polyacrylamide beads. Controlled introduction of functional groups for the preparation of special-purpose, biochemical adsorbents. Biochemistry. 1969 Oct;8(10):4074–4082. doi: 10.1021/bi00838a026. [DOI] [PubMed] [Google Scholar]
- Iwai K., Hayashi H., Ishikawa K. Calf thymus lysine- and serine-rich histone. 3. Complete amino acid sequence and its implication for interactions of histones with DNA. J Biochem. 1972 Aug;72(2):357–367. doi: 10.1093/oxfordjournals.jbchem.a129911. [DOI] [PubMed] [Google Scholar]
- Kamen R. Characterization of the subunits of Q-beta replicase. Nature. 1970 Nov 7;228(5271):527–533. doi: 10.1038/228527a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miwa M., Sugimura T., Inui N., Takayama S. Poly(adenosine diphosphate ribose) synthesis during the cell cycle of transformed hamster lung cells. Cancer Res. 1973 Jun;33(6):1306–1309. [PubMed] [Google Scholar]
- Nishizuka Y., Ueda K., Honjo T., Hayaishi O. Enzymic adenosine diphosphate ribosylation of histone and poly adenosine diphosphate ribose synthesis in rat liver nuclei. J Biol Chem. 1968 Jul 10;243(13):3765–3767. [PubMed] [Google Scholar]
- Nishizuka Y., Ueda K., Nakazawa K., Hayaishi O. Studies on the polymer of adenosine diphosphate ribose. I. Enzymic formation from nicotinamide adenine dinuclotide in mammalian nuclei. J Biol Chem. 1967 Jul 10;242(13):3164–3171. [PubMed] [Google Scholar]
- Nishizuka Y., Ueda K., Nakazawa K., Hayaishi O. Studies on the polymer of adenosine diphosphate ribose. I. Enzymic formation from nicotinamide adenine dinuclotide in mammalian nuclei. J Biol Chem. 1967 Jul 10;242(13):3164–3171. [PubMed] [Google Scholar]
- Nishizuka Y., Ueda K., Yoshihara K., Yamamura H., Takeda M., Hayaishi O. Enzymic adenosine diphosphoribosylation of nuclear proteins. Cold Spring Harb Symp Quant Biol. 1969;34:781–786. doi: 10.1101/sqb.1969.034.01.088. [DOI] [PubMed] [Google Scholar]
- Okayama H., Edson C. M., Fukushima M., Ueda K., Hayaishi O. Purification and properties of poly(adenosine diphosphate ribose) synthetase. J Biol Chem. 1977 Oct 25;252(20):7000–7005. [PubMed] [Google Scholar]
- Ord M. G., Stocken L. A. Adenosine diphosphate ribosylated histones. Biochem J. 1977 Mar 1;161(3):583–592. doi: 10.1042/bj1610583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otake H., Miwa M., Fujimura S., Sugimura T. Binding of ADP-ribose polymer with histone. J Biochem. 1969 Jan;65(1):145–146. [PubMed] [Google Scholar]
- PHILLIPS D. M. The presence of acetyl groups of histones. Biochem J. 1963 May;87:258–263. doi: 10.1042/bj0870258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- RASMUSSEN P. S., MURRAY K., LUCK J. M. On the complexity of calf thymus histone. Biochemistry. 1962 Jan;1:79–89. doi: 10.1021/bi00907a013. [DOI] [PubMed] [Google Scholar]
- Roberts J. H., Stark P., Smulson M. Stimulation of DNA synthesis by adenosine diphosphoribosylation of HeLa nuclear proteins during the cell cycle. Biochem Biophys Res Commun. 1973 May 1;52(1):43–50. doi: 10.1016/0006-291x(73)90951-0. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Wiebers J. L., Gilham P. T. Studies on the interactions of nucleotides, polynucleotides, and nucleic acids with dihydroxyboryl-substituted celluloses. Biochemistry. 1972 Sep 12;11(19):3623–3628. doi: 10.1021/bi00769a020. [DOI] [PubMed] [Google Scholar]
- Schott H., Rudloff E., Schmidt P., Roychoudhury R., Kössel H. A dihydroxyboryl-substituted methacrylic polymer for the column chromatographic separation of mononucleotides, oligonucleotides, and transfer ribonucleic acid. Biochemistry. 1973 Feb 27;12(5):932–938. doi: 10.1021/bi00729a022. [DOI] [PubMed] [Google Scholar]
- Smith J. A., Stocken L. A. Chemical and metabolic properties of adenosine diphosphate ribose derivatives of nuclear proteins. Biochem J. 1975 Jun;147(3):523–529. doi: 10.1042/bj1470523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. A., Stocken L. A. Identification of poly (ADP-ribose) covalently bound to histone F1 in vivo. Biochem Biophys Res Commun. 1973 Sep 5;54(1):297–300. doi: 10.1016/0006-291x(73)90922-4. [DOI] [PubMed] [Google Scholar]
- Ueda K., Omachi A., Kawaichi M., Hayaishi O. Natural occurrence of poly(ADP-ribosyl) histones in rat liver. Proc Natl Acad Sci U S A. 1975 Jan;72(1):205–209. doi: 10.1073/pnas.72.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wong N. C., Poirier G. G., Dixon G. H. Adenosine diphosphoribosylation of certain basic chromosomal proteins in isolated trout testis nuclei. Eur J Biochem. 1977 Jul 1;77(1):11–21. doi: 10.1111/j.1432-1033.1977.tb11635.x. [DOI] [PubMed] [Google Scholar]
- Yoshihara K., Tanigawa Y., Burzio L., Koide S. S. Evidence for adenosine diphosphate ribosylation of Ca2+, Mg2+-dependent endonuclease. Proc Natl Acad Sci U S A. 1975 Jan;72(1):289–293. doi: 10.1073/pnas.72.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara K., Tanigawa Y., Koide S. S. Inhibition of rat liver Ca2+, Mg2+-dependent endonuclease activity by nicotinamide adenine dinucleotide and poly (adenosine diphosphate ribose) synthetase. Biochem Biophys Res Commun. 1974 Jul 24;59(2):658–665. doi: 10.1016/s0006-291x(74)80030-6. [DOI] [PubMed] [Google Scholar]