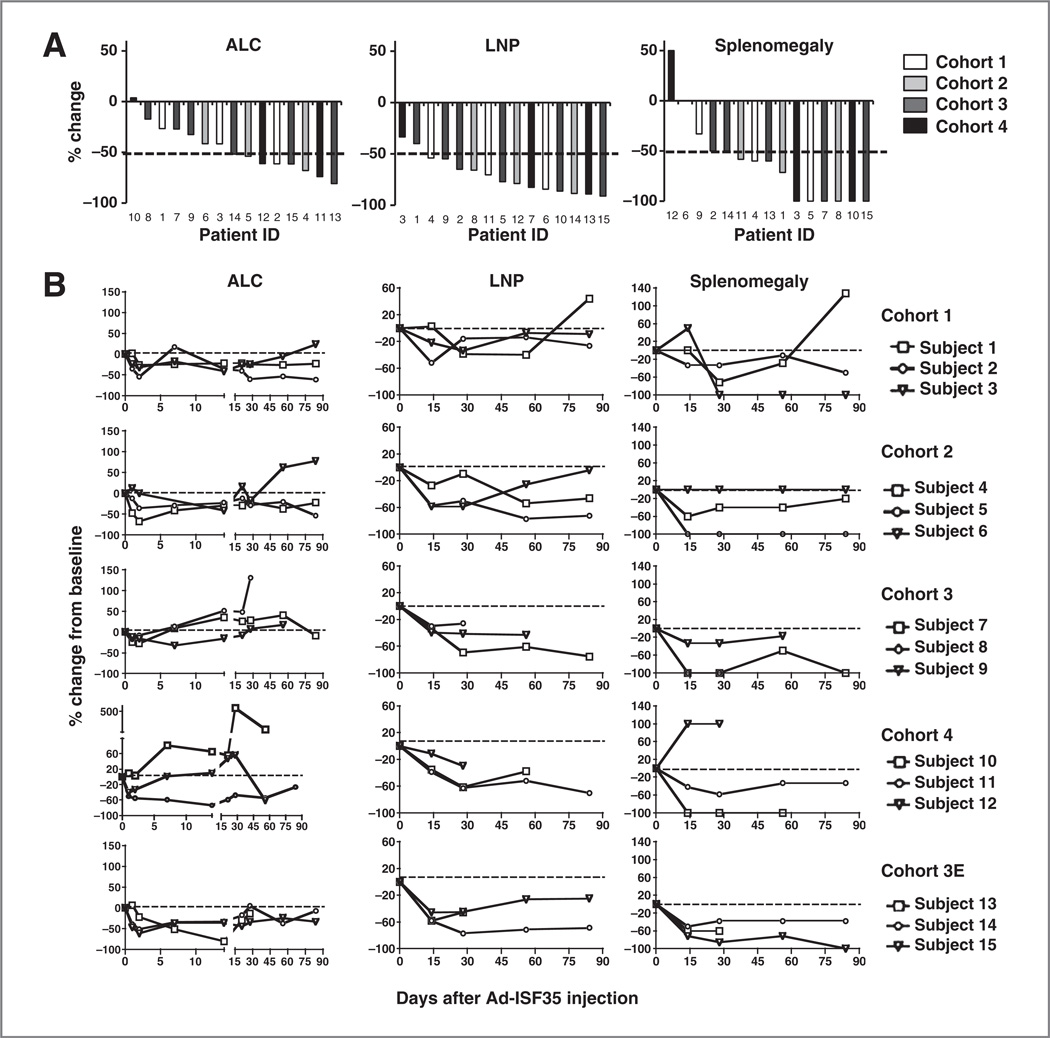
Figure 3.
Clinical parameters of patients who received Ad-ISF35 IDI. Patients with CLL were enrolled in 4 cohorts using a 3 + 3 dose-escalation design with doses of Ad-ISF35 ranging from 1 × 1010 to 33 × 1010. A, ALC, bidimensional lymph node product (LNP) of measurable and palpable lymph nodes, and palpable spleen size (below the left costal margin) were recorded from day 0 (Ad-ISF35 IDI) to day 90. Data were normalized to day 0 and the best response change is defined as the maximum reduction/minimum increase from baseline. The dotted line indicates 50% reduction. B, ALC, LNP, and splenomegaly are shown overtime. Cohort 3E corresponds to the extension of cohort 3 after toxicities in cohort 4 were observed. The dotted line indicates 0% change from baseline.