Table 2.
Tin-Mediated Radical Addition to Chiral N-Acylhydrazone 10a in the Presence of ZnCl2
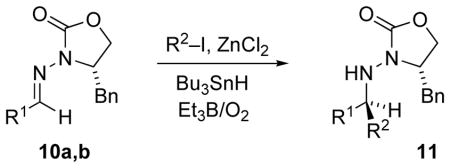
| |||
|---|---|---|---|
| Entry | R1 | R2 | Product, Yieldb(dr) |
| 1 | Et (10a) | i-Pr | 11a, 60% (99:1) |
| 2 | Et (10a) | c-C5H9 | 11b, 59% (96:4) |
| 3 | Et (10a) | c-C6H11 | 11c, 28% (97:5) |
| 4 | Et (10a) | t-Bu | 11d, 54% (95:5) |
| 11 | Ph (10b) | i-Pr | 11e, 42% (99:1) |
| 12 | Ph (10b) | c-C5H9 | 11f, 59% (96:4) |
| 13 | Ph (10b) | c-C6H11 | 11g, 30% (99:1) |
| 14 | Ph (10b) | t-Bu | 11h, 83% (93:7) |
Reaction conditions: Bu3SnH (5 equiv) and O2 (7mL/mmol) by syringe pump, i-PrI (10 equiv), Et3B (10 equiv), and Lewis acid (2 equiv), 2:1 CH2Cl2/ether, −78°C → rt.
Recovered hydrazone, %.
Isolated yield, %.
Not determined.
