Table 4.
Mn-Mediated Radical Additions to N-Acylhydrazones.
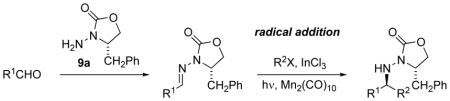
| |||||
|---|---|---|---|---|---|
| entry | aldehyde (or acetal) | yield, hydrazonea | halide R2X | yield, radical additionb | dr |
| 1 | CH3CH2CHO | 81% | CH3CH2I | 85% | - |
| 2 | CH3I | 48%c,d (S) | 95:5e | ||
| 3 | n-PrI | 66% (R) | 94:6e | ||
| 4 | n-BuI | 78% (R) | 95:5e | ||
| 5 | n-C5H11I | 79% (R) | 96:4e | ||
| 6 | i-BuI | 54%c (R) | 95:5f | ||
| 7 | i-PrI | 75% (R) | 95:5f | ||
| 8 | ClCH2I | 63% (R) | 93:7e | ||
| 9 | Cl(CH2)3I | 52% (R) | 96:4f | ||
| 10 | Cl(CH2)4I | 55% (R) | 96:4e | ||
| 11 | Cl2CHBr | 38%c,d (R) | 98:2f | ||
| 12 | CH3CHO | 66% | CH3CH2I | 66% (R) | 95:5e |
| 13 | n-PrCHO | 87% | 63% (S) | 95:5e | |
| 14 | n-BuCHO | 89% | 72% (S) | 97:3e | |
| 15 | n-C5H11CHO | 88% | 77% (S) | 97:3e | |
| 16 | i-BuCHO | 85% | 65% (S) | 95:5f | |
| 17 | ClCH2CH(OMe)2 | 85% | 57% (S) | 93:7e | |
| 18 | Cl(CH2)3CHO | 95% | 60% (S) | 93:7f | |
| 19 | Cl(CH2)4CHO | 89% | 62% (S) | 97:3e | |
| 20 | Cl2CHCH(OEt)2 | 54% | 34%c (S) | 89:11f | |
Reaction conditions: (1) Aldehyde or acetal (5–10 equiv), 9a, p-toluenesulfonic acid, CH2Cl2, rt. (2) Hydrazone in deoxygenated CH2Cl2 (0.1 M), InCl3 (2.2 equiv), Mn2(CO)10 (1–2 equiv), R2X (10 equiv), hν (300 nm, pyrex), 1–2 d, ca. 35 °C.
Isolated yield.
Isolated yields of purified diastereomer mixtures. R or S denotes the configuration of the new stereogenic center. Addition of methyl iodide gives opposite configurations due to the lower priority of the methyl ligand.
20 equiv of R2X was used.
1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) was used in removal of Mn byproducts.
Ratio by HPLC (Chiralcel OD, 2-PrOH/hexane).
Ratio by 1H NMR.
