Table 7.
Varying the radical precursors and acceptors in Cu(II) catalyzed addition to N-acylhydrazones.
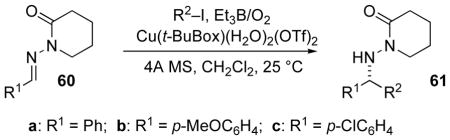
| |||||
|---|---|---|---|---|---|
| entry | halide | hydrazone | catalyst load | % yieldb | % eec |
| 1 | iPrI | 60a | 1 equiv | 66 | 95 |
| 2 | i-PrI | 60b | 1 equiv | 46 | 90 |
| 3 | i-PrI | 60c | 1 equiv | 53 | 81 |
| 4 | EtId | 60a | 1 equiv | 88 | 83 |
| 5 | c-C5H9Id | 1 equiv | 86 | 84 | |
| 6 | c-C6H11Id | 1 equiv | 84 | 89 | |
| 7 | ClCH2Id | 1 equiv | 44e | 95 | |
| 8 | iPrI | 0.5 equiv | 71 | 81 | |
| 9 | iPrI | 0.2 equiv | 83 | 58 | |
| 10 | iPrI | 0.1 equiv | 74 | 46 | |
Reaction conditions: see Table 6.
Isolated yield, %.
Enantiomeric excess, % (hexane:2-propanol, Chiralcel OD or AD).
10 equiv of alkyl halide was used.
56% recovery of unreacted hydrazone.
