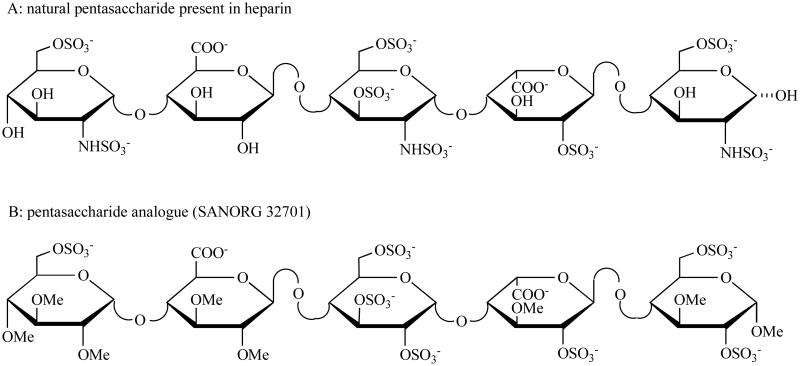
Fig. (1). Molecular structures of the pentasaccharide sequence present in heparin and its synthetic derivative.
Heparin is composed of alternating hexosamine (α-D-glucosamine) and hexuronic acid (α-L-iduronic acid or β-D-glucuronic acid) residues, joined as a disaccharide unit by (1,4)-glycosidic linkages. The natural pentasaccharide binds to antithrombin with a high affinity. A synthetic form of pentasaccharide (SR 90107A/Org 31540, fondaparinux) is the alpha methyl glycoside of the natural pentasaccharide, which exactly reproduces this unique sequence. SANORG 32701 is the synthetic analogue of natural pentasaccharide with the similar biologic effects.