Abstract
GABAA receptors (GABARs) have long been the focus for acute alcohol actions with evidence for behaviorally relevant low millimolar alcohol actions on tonic GABA currents and extrasynaptic α4/6, δ, and β3 subunit-containing GABARs. Using recombinant expression in oocytes combined with two electrode voltage clamp, we show with chimeric β2/β3 subunits that differences in alcohol sensitivity among β subunits are determined by the extracellular N-terminal part of the protein. Furthermore, by using point mutations, we show that the β3 alcohol selectivity is determined by a single amino acid residue in the N-terminus that differs between GABAR β subunits (β3Y66, β2A66, β1S66). The β3Y66 residue is located in a region called “loop D” which in γ subunits contributes to the imidazobenzodiazepine (iBZ) binding site at the classical α+γ2− subunit interface. In structural homology models β3Y66 is the equivalent of γ2T81 which is one of three critical residues lining the benzodiazepine binding site in the γ2 subunit loop D, opposite to the “100H/R-site” benzodiazepine binding residue in GABAR α subunits. We have shown that the α6R100Q mutation at this site leads to increased alcohol-induced motor in-coordination in alcohol non-tolerant rats carrying the α6R100Q mutated allele. Based on the identification of these two amino acid residues α6R100 and β66 we propose a model in which β3 and δ containing GABA receptors contain a unique ethanol site at the α4/6+β3− subunit interface. This site is homologous to the classical benzodiazepine binding site and we propose that it not only binds ethanol at relevant concentrations (EC50– 17 mM), but also has high affinity for a few selected benzodiazepine site ligands including alcohol antagonistic iBZs (Ro15-4513, RY023, RY024, RY80) which have in common a large moiety at the C7 position of the benzodiazepine ring. We suggest that large moieties at the C7-BZ ring compete with alcohol for its binding pocket at a α4/6+β3− EtOH/Ro15-4513 site. This model reconciles many years of alcohol research on GABARs and provides a plausible explanation for the competitive relationship between ethanol and iBZ alcohol antagonists in which bulky moieties at the C7 position compete with ethanol for its binding site. We conclude with a critical discussion to suggest that much of the controversy surrounding this issue might be due to fundamental species differences in alcohol and alcohol antagonist responses in rats and mice.
Keywords: Alcohol, Alcohol antagonist, Benzodiazepine, GABAA receptor, Tonic current
Introduction
Despite the fact that alcohol is the most widely used recreational drug, the mechanisms whereby ethanol (EtOH) leads to alterations in human brain function are unclear and remain controversial. Over the last four decades evidence from pharmacological and behavioral studies has implicated the GABAergic system and inhibitory GABARs as important mediators of acute alcohol actions. It was, for example, shown that the GABA analog and GABAR agonist muscimol potentiated sedative EtOH actions, while the opposite effect was observed with the administration of general GABAR receptor blockers bicuculline and picrotoxin [1].
Particular interesting are imidazobenzodiazepine (iBZ) compounds like Ro15-4513, initially discovered for its alcohol counteracting actions by Hofmann La Roche scientists [2] and other closely related i-BZ compounds (for review see Wallner and Olsen [3]). In marked contrast to other classical BZ agonists (e.g., diazepam) that enhance alcohol actions, Ro15-4513 and closely related iBZ analogs have potent alcohol antagonistic actions and lead to a drastic decrease of alcohol intoxication in rats, in particular at low alcohol doses [4, 5]. Another piece of direct evidence that alcohol might act on GABA receptors and on BZ binding sites is a polymorphism in the cerebellar granule cell α6 subunit α6-R100Q found in so called alcohol non-tolerant (ANT) rats. This polymorphism was initially discovered by showing that diazepam displaced [3H]Ro15-4513 in cerebellar granule cells of ANT rats but not in alcohol tolerant (AT) animals [6]. This and later work indicated that the α6R100Q mutation confers sensitivity to classical benzodiazepines like diazepam to α6R100Qβγ2 receptors [7], and explains not only the increased behavioral diazepam sensitivity of α6R100Q animals but also why diazepam displaces [3H]Ro15-4513 in ANT rats [6]. It was more than a decade later that we showed that the increased motor-impairing effects of EtOH in ANT α6R100Q rats can be explained by increased alcohol sensitivity of tonic currents in cerebellar granule cells. We showed that the α6R100Q mutation further increased the already high alcohol sensitivity of α4/6βδ receptors but only when expressed with the β3 subunit, not with the β2 subunit [8]. The α4/6R100 residue is a histidine residue in diazepam-sensitive α1/2/3/5γ2 receptors and arginine at the α4/6-100 position renders α4/6βγ2 receptors insensitive to classical benzodiazepines like diazepam [9, 10].
While it has been shown that alcohols (EtOH and other longer chain alcohol anesthetics) can act on GABAA receptors (GABARs) at sites defined by transmembrane amino acid residues like β265, also crucial for the actions of anesthetics like etomidate and propofol, these effects require very high, potentially deadly (anesthetic) concentrations of ethanol of 100 mM or higher [11]. In contrast pronounced intoxicating ethanol effects are observed already at doses at and below 10 mM (the blood alcohol driving limit in most EU countries). It was therefore exciting to find that recombinantly expressed extrasynaptic δ subunit-containing GA-BARs are enhanced by relevant low alcohol concentrations [12, 13], and there is now essentially agreement that in δ subunit expressing neurons tonic extrasynaptic GABA currents are indeed sensitive to such behaviorally relevant low EtOH concentrations [8, 14–21]. Other detailed positive evidence for the involvement of δ receptors in acute and chronic alcohol actions is the rather remarkable down-regulation of cell surface δ receptors after acute and chronic alcohol administration in rat models, which may not only explain phenomena like acute alcohol tolerance, but also cross-tolerance to GABA-receptor active drugs like anesthetics and benzodiazepines [22–25]. Such plasticity and the underlying compensatory mechanisms might help explain why the alcohol phenotypes of α4, α6 and δ subunit global knock-out mice are less evident than expected from the simple removal of subunits [26, 27].
Here we show that the higher EtOH sensitivity of β3 subunit-containing receptors is determined by differences in amino acid residues at position β66 in the N-terminus. The β66 position is homologous to a residue in the γ2 subunit (γ2T81) that determines iBZ-sensitivity at the classical α+/γ2− subunit interface [28, 29]. Based on these findings we propose a model in which the EtOH/Ro15-4513 binding site on δ-containing receptors is located at the α+β3− subunit interface where both the α6R100 and β3Y66 sites might be able to directly contribute to the EtOH/Ro15-4513 binding site. We suggest a model where the EtOH binding pocket is located right between the α4/6R100Q residue and β3-Y66 at the α+/β3−. We think that this and perhaps other similar EtOH/iBZ binding sites provide a plausible molecular explanation for important low dose alcohol actions in mammals.
Methods
The rat GABAR cDNA clones were as previously described [13]. Chimeric constructs were made using a PCR overlap extension method [30] and point mutations were introduced using a PCR amplification based method (QuikChange Stratagene). mRNAs were transcribed from linearized template plasmids using the mMessage-mMachine kits (Ambion), transcripts were purified by LiCl precipitation and transcript quality and concentration were estimated by photometry and gel electrophoresis. Oocytes were injected with 0.4 ng α and β subunit cRNA and 2 or 4 ng of δ cRNA and measured 3–10 days after injection (Fig. 1).
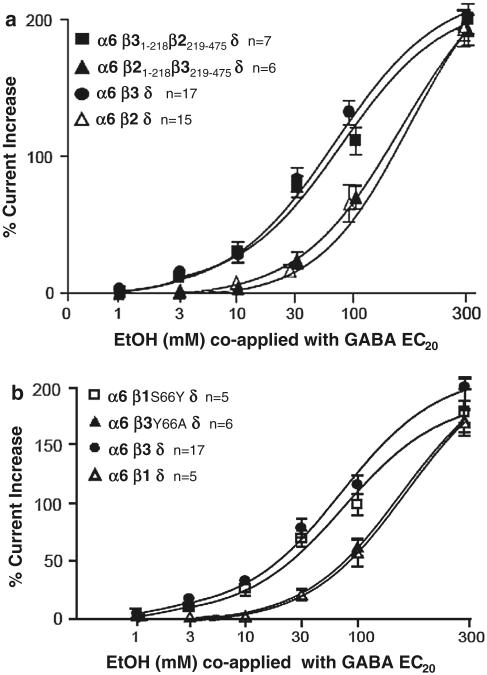
Fig. 1.
Localization of amino acid residues responsible for differences in alcohol sensitivity between β3 and β1,2 subunits. The upper panel (a) alcohol sensitivity of chimeras made by swapping the β2 N-terminal domain and the β3 C-terminal domain. Receptors containing the N-terminal domain of the δ subunit (residues 1–128) and the C-terminal (transmembrane) domain of γ2 (filled square) responded to co-application of GABA (EC20) with EtOH in a dose-dependent manner (threshold response at 3 mM EtOH, almost identical to wildtype α6β3δ GABARs). In contrast α6β2(N1–218)β3(C219–475)δ-containing receptor threshold response is at 30 mM EtOH, much like that of wild-type α6β2δ GABARs (filled triangle). b Point mutations identify residue Y66 in β3 as critical for low dose ethanol effects. Much like β2-containing receptors, β1-containing GABARs do not respond to ethanol below 30 mM (open triangle) whereas β3-containing GABARs respond to 3 mM. A point mutation β1S66Y (open square) makes α6β1δ gain the ethanol sensitivity of β3-containing receptors (filled circle). The reverse mutation from tyrosine (Y, found in β3) to alanine (A, found in β2) causes a tenfold loss of ethanol sensitivity (filled triangle) (also, see alignment in Fig. 3d)
GABAR currents were measured in Xenopus laevis oocytes with an Axoclamp 2A amplifier (Axon Instruments) in the two electrode voltage clamp configuration. Electrodes were filled with 3 M KCl and had resistances between 0.5 and 1.5 Mω when measured dipped in the bath solution. The oocyte chamber was continuously perfused with ND96 bath solution (composition 96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5 mM HEPES/pH7.5) with drugs and treatments mentioned. Solution exchanges were triggered with a programmable valve bank switching a three way solenoid valve, and bath volume exchange times were in the range of 1–3 s. Currents were measured at −80 mV, where GABA applications evoked an inward current in oocytes. Ethanol enhancement was measured as an increase in GABA EC20 responses and curve fits for the EtOH dose-response curves were generated by non-linear sigmoidal dose-response curves as described [13]. GABA EC50 values for mutants and both chimaeras were similar to GABA EC50 of WT receptors. The GABA receptor structure shown in Fig. 2 is a homology model using the acetylcholine binding protein (AChBP) structural coordinates [31] and is based on protein sequence alignments. The homology model was constructed using the Deep View homology modeling program [32]. While alignments of GABAR with AChBP can be ambiguous due to the low sequence conservation, the protein sequences of GABA receptors subunits are highly conserved and therefore the alignments among GABAR subunits shown in Fig. 3c, d are unambiguous.
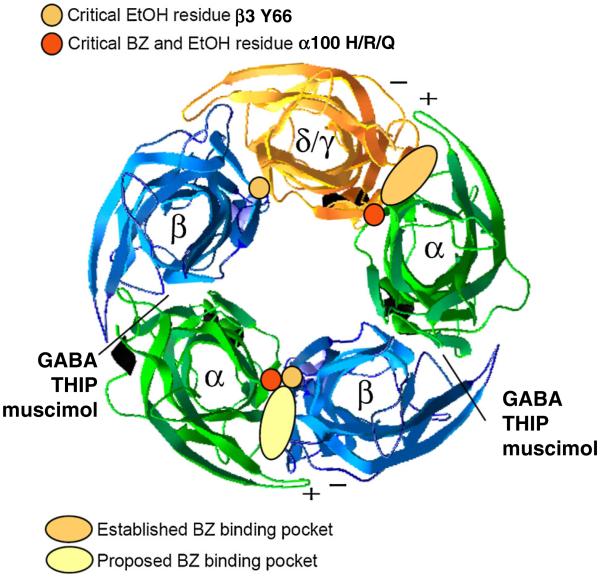
Fig. 2.
Proposed sites of EtOH and BZ actions. The conventional BZ binding pocket is at the α and γ interface. Of critical importance at this interface is the histidine (arginine in α4 & α6) residue (red dot, α subunit), which is necessary for sensitivity to classical BZs (see alignment in Fig. 3c). In pale yellow is our proposed new BZ site at the α+/β− interface which we suggest to be important for low dose ethanol actions on δ-GABAARs, because of the critical role of the β3Y66 residue. Another potential site for ethanol effects would be the α +/δ− interface in δ containing GABARs
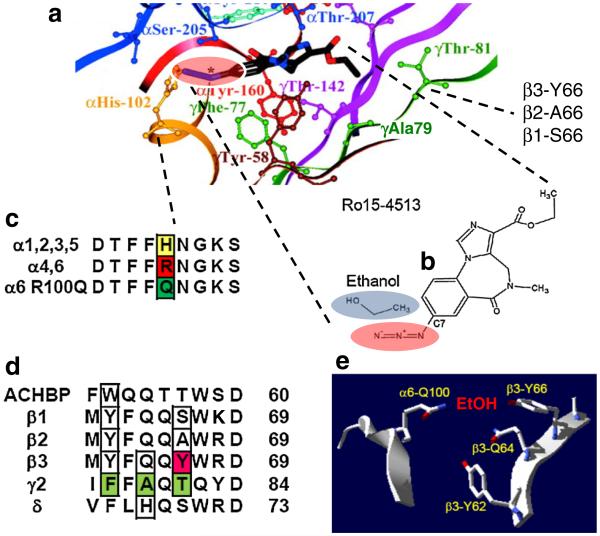
Fig. 3.
Structural alcohol site model. a Ro15-4513 docking model in the classical α+γ2− BZ binding site (adapted from Fig. 10 in Sawyer et al, JBC 2002 [40]. The docked Ro15-4513 molecule is in a stick representation with the −N=N = N+ group indicated by an asterisk (structure of Ro15-4513 is enlarged in b). Colored yellow in a is part of α “loop A” with the BZ critical H102 residue (see alignment in c with H (yellow), R (red) and the α6R100Q (green). Shown in green in a is γ2 “loop D” forming a beta sheet structure with three amino acid residues (F(Phe)77, A(Ala)79, T(Thr)81) pointing into and lining the benzodiazepine binding pocket. Panel c shows a sequence alignment of the critical BZ residue α1,2,3,5 H, α4,6 R and a glutamine (Q) in the α6 subunit in alcohol non-tolerant (ANT) rats. Panel d shows a protein sequence alignment of loop D (region shown in green in a). AChBP is AChBP, on which the structural models are built on. Boxed in this alignment are “alcohol residues” β66 and the three critical “loop” D γ residues F77, A79 and T81. Also boxed is residue δH68 that confers diazepam sensitivity to α4β3δ receptors when changed to A, the residue present in γ2 at the homologous position [50] and a tryptophane residue in the acetylcholine/nicotine binding site. Panel e shows a structural rendering of a possible proposed EtOH binding pocket with EtOH between α6R100Q and β3− Y66 residues at the α+β− subunit interface; this is a possibility if—the fairly long—α6-100R/Q and the β3-66Y amino-acid side-chains are fully extended towards each other
Results
Alcohol Sensitivity Differences in β Subunits are Determined by Amino Acid Residue β66
We have previously reported that alcohol enhancement of GABARs is dependent on the GABAA receptor subtypes studied. Alcohol sensitivity at concentrations at and below 17 mM (the legal blood alcohol driving limit in most US states) requires the δ as well as the β3 subunit. When expressed with α4 or α6 and δ subunits, both β1 and β2 subunit-containing receptors are much less alcohol-sensitive, with little enhancement at alcohol concentrations below 30 mM [8, 13]. To identify the molecular determinants which confer differential sensitivity in β3 versus β2 subunits we made chimeric β2β3 and β3β2 constructs by swapping the N-terminal extracellular domains and C-terminal transmembrane region containing parts. Co-expression together with the α6 and δ subunit in Xenopus oocytes showed that high alcohol sensitivity requires the presence of the β3 subunit N-terminal domain (β3N1-218), rather than the C-terminus (see Fig. 1a), which would have been expected from previous molecular studies which have identified critical alcohol/anesthetic residues in GABAA and glycine receptors [11]. Experiments with recombinant β1-containing α6β1δ GABAARs showed an EtOH dose response much like those containing the α6β2δ subunits (see Fig. 1b). To identify the amino acids in β subunits that might mediate differences in EtOH sensitivity we compared β-subunit protein sequence alignments. Since the three β subunits are very similar and differ only in a few amino acids at the N-terminus we noted that amino acid position β66 differed between the three β subunits, with β1S66, β2A66 and β3Y66 (see alignment in Fig. 3d) in an otherwise highly conserved region. Serine in β1S66 and alanine in β3A66 are very similar in size and chemistry, whereas tyrosine in β3Y66 contains a long bulky side chain with an aromatic ring. In addition, when comparing multiple sequence alignments of GABA subunits it caught our attention that position β66 is homologous to the amino acid residue γ2T81 which is critical for iBZ binding in the γ2 subunit [28, 29, 33]. To test the hypothesis that amino acid residue β3Y66 determines low dose EtOH sensitivity, we made two point mutations. A mutation in the β1 subunit where we replaced S66 with Y66 found in the β3 subunit (β1S66Y) led to a gain of alcohol sensitivity in α6β1S66Yδ receptors and showed alcohol sensitivity similar to WT α6β3δ receptors. In contrast, replacing tyrosine Y66 in β3 with alanine A66, the residue found in β2 at the same position (β3Y66A), leads to reduced alcohol sensitivity, and alcohol enhancement very similar to what we see with α6β1δ (Fig. 1b) and what we previously reported with recombinant α6β2δ GABARs [8].
GABA Receptor Model Showing That the Positions α6R100Q and β3Y66 Important for Alcohol Sensitivity are Located at the α+/β− Subunit Interface
One of the best studied residues in the BZ binding pocket is a histidine (H) residue that is conserved in α1,2,3,5, but is an arginine (R) in α4 and α6 (see alignment in Fig. 3c). The arginine at this position makes α4 and α6 subunits (when expressed with β and γ2) insensitive to classical BZ agonists and this changes the efficacy of Ro15-4513 (agonist on receptors containing an “R”; inverse agonist on receptors containing an “H” [9]). This difference has been utilized to generate benzodiazepine-insensitive mice through knock-in point mutations [10, 34, 35]. The homologous residue in the α6 subunit is polymorphic in laboratory rats where this amino acid can be an arginine (α6R100) as well as glutamine (α6Q100). The α6R100Q allele has been enriched in three independent breeding studies that selected rats for behavioral alcohol sensitivity [36–38]. We showed that the R100Q mutation leads to increased alcohol sensitivity in vivo in rats carrying this mutation, showed increased alcohol enhancement of tonic currents in cerebellar granule cells harboring the mutated α6 subunit, and increased EtOH-sensitivity of recombinant α6R100Qβ3δ receptors [8]. The approximate locations of amino acid residues β3Y66 (yellow dots) and α6R100 (red dots) are indicated in the structural homology model based on the structure of the AChBP which provides a structural template for the extracellular domains of GABARs without the transmembrane regions. Because each pentamer in this model contains two identical α6 and β3 subunits, each mutation is present at two positions in the pentamer. The α6R100Q position is present at the α6+δ− and at the α6+β3− interface, whereas the β3Y66 position is present at the δ+β3− and α6+β3− interface. Only at the α+β− interface the α6R/Q100 and the β3Y66 residues are found at the same interface where they could contribute to a unique alcohol/BZ-binding pocket. Note that in this proposed α+β− binding pocket the α+ interface is the same as in the classical α+γ− BZ binding pocket, but contains a unique β− interface which would to a large extent determine the pharmacology of such a binding site. Note that the two extracellular β+α− interfaces in this homology model are the binding sites for GABA (and GABA analogs like THIP and muscimol) and therefore such EtOH/BZ-ligand binding pockets at subunit interfaces are essentially modified GABA binding sites in these heteromeric receptors, as noted earlier for the traditional BZ binding pockets [39].
Discussion
Proposed Structural Model of the Suggested EtOH Ro15-4513 Binding Site
Figure 3a is a structural docking model reproduced from Sawyer et al. [40] with Ro15-4513 (stick representation) docked into the classical BZ site at the α+γ2− subunit interface of a GABAR/AChBP homology model. A very similar model has been published based on data showing that amino acids γ2A79 and γ2T81 are particularly important for iBZ binding since they likely make contact with the unique imidazo ring and the carboxyethylester moiety (position 3)’ in iBZs [28, 29]. Note that the γThr81 (green in Fig. 3a) aligns with, and therefore corresponds to, the β66 position that we identified here as critical for low concentration EtOH actions; the α6R100Q position corresponds to αHis-102 (yellow in Fig. 3a, c). The proximity of moieties at the C7 benzodiazepine position (which contains an azido group in Ro15-4513 and other bulky moieties in iBZ alcohol antagonists) to this histidine in the α1 subunit has been experimentally verified using covalent labeling techniques using a diazepam analog with a cysteine reactive group at the C7 position and α1H101C [41].
Note that flumazenil, which does not show alcohol antagonistic actions [5], and Ro15-4513 are identical, except that flumazenil contains a much smaller fluorine atom at the C7 position of the BZ-structure (in place of the azido-group in Ro15-4513 marked in red in Fig. 3b) and therefore this position must be critical for the behavioral alcohol antagonism of iBZs. Since flumazenil can bind to α4β3δ receptors with high affinity [42] and flumazenil can inhibit Ro15-4513’s alcohol antagonism on recombinant receptors [43] we proposed that both flumazenil and alcohol fit next to each other in this binding site, but both EtOH and flumazenil compete with Ro15-4513 for an overlapping binding pocket, thus providing a plausible molecular explanation why excess flumazenil can prevent Ro15-4513 from acting as an alcohol antagonist in vivo in rats [4, 44]. It is noteworthy in this regard that other iBZs (e.g., RY024 and RY008) have been described as alcohol antagonists in rats, both containing bulky side chains at the C7 positions, but are otherwise identical with flumazenil and Ro15-4513 [3, 45, 46].
Figure 3e shows a simplified rendering of this structural model where the side chains α6Q100 and β3Y66 are extended towards each other. This is to demonstrate that two long amino acid side chains can span considerable distances in a protein structure. Note that the side chain of the amino acid arginine at α6R100Q is even longer than glutamine (Q). Therefore there is the interesting possibility that these two amino acid residues at positions α6R\Q100 and β3Y66 could directly contact the alcohol molecule and participate in forming the alcohol binding pocket, even though the back-bone positions are apparently not that close to each other in the structural homology model. In addition, there is the possibility that incorporation of the delta subunit could lead to allosteric structural change in the backbone, so that those two critical positions move closer to each other. Such a structural alcohol/Ro15-4513 site model provides a plausible explanation for the initially puzzling observation that the α6R100Q mutation increases alcohol sensitivity only in αβ3δ receptors but not when expressed with β2 [8]. If the much shorter amino acids serine and alanine found at the β1/2-66 position do not support a high affinity alcohol binding site, we would predict that only β3 subunit-containing receptors would be responsible for Ro15-4513’s reversible action on low dose EtOH effects, and that other alcohol effects are mediated by actions on other receptors, including higher dose action at the anesthetic EtOH sites in GABAR transmembrane regions. These transmembrane alcohol sites apparently do not have much subtype specificity [11, 47] and likely make important contributions to Ro15-4513-insensitive behavioral EtOH actions at higher EtOH doses.
Our BZ/EtOH binding site model at the α+β− subunit interface provides a detailed molecular explanation for our previous findings that the behavioral alcohol antagonist Ro15-4513 specifically reversed ≤30 mM EtOH actions on these receptors [43] and that expressed and immune-purified native α4β3δ receptors bind Ro15-4513 (and a small selected group of BZ-site ligands including the β-carboline β-CCE) with high affinity.
While we think that all of this provides possible and plausible explanations for specific alcohol action on a unique BZ-site, many important open questions remain. One of these is whether in native δ-containing GABAR the δ subunit indeed replaces the γ subunit as shown in Fig. 2. There are recent reports that δ subunit over-expression and expression of concatenated receptors leads to heterogeneous subunit stoichiometries and unusual wheel arrangements, including pentamers with more than one δ included [48, 49]. However, some of these complexes may not produce functional channels or produce partially functional abnormal channels, and since there is no evidence that these receptors resemble native receptors in pharmacological properties (e.g., EtOH, THIP sensitivity), we think it remains reasonable to assume a subunit arrangement of native GABAR where the δ subunit replaces the γ subunit in functional/native receptor pentamers (as in Fig. 2).
It seems like an apparent contradiction of this proposed alcohol site model that the high alcohol sensitivity of α4/6β3δ GABARs is not observed when the δ subunit is replaced with the γ2 subunit in α6β3δ/γ2 receptors [8, 13] when neither the δ nor the γ2 subunit contributes to the proposed α+β− EtOH binding site in the structural model shown in Fig. 2. However, analogous to allosteric modulation in enzymes, the incorporation of the δ or γ2 subunit could lead to changes in ligand affinities at distant sites through changes in protein conformation. In fact, we have recently shown that δ-subunit expression leads to a dramatic three orders of magnitude increase in THIP sensitivity of recombinantly expressed receptors [50], which suggests that indeed δ subunit incorporation can lead to dramatic changes in ligand affinities at subunit interfaces by allosterically changing the conformation of the protein. Therefore we propose that the δ subunit is required for high EtOH and Ro15-4513 affinity at the proposed α+β− binding site. Such a concept of allosteric changes in ligand affinities in GABARs explains how anesthetic binding at transmembrane binding sites increases GABA sensitivity and may also help to explain the action of classical BZ agonists, which when bound to the classical α+γ− binding site, might stabilize a protein conformation that increases the affinity of GABA in its binding site. In addition, this alcohol site model opens the possibility that other β3 subunit containing GABARs could under the right conditions, e.g. through modulatory protein binding or binding of modulatory ligands, stabilize the alcohol conformation of αβ3γ receptors and render them sensitive to low doses of EtOH.
In support of the notion that BZ-site ligands can bind to other (homologous) subunit interfaces, there are reports that such novel α+β− BZ-like binding sites do exist, including a recent report in δ-containing receptors in this special issue of Neurochemical Research [51]. In α1β3 and α1β3γ2 GA-BARs the extracellular α1+β3− site has been shown to be modulated by the BZ site ligand CGS9895 [52]. In addition, it has been shown that flurazepam can interact at concentrations >10 μM with the extracellular α1+β2− interface and thereby prevent modulation through the classical benzodiazepine site [53]. It has been argued that such α+β− BZ-like binding sites might account, at least in part, for other low affinity benzodiazepine sites that have been described [53], but have been thought to indicate unique low affinity BZ sites in transmembrane regions [54].
The actions of Ro15-4513 as an alcohol antagonist have been in the past [55] and still remain a highly contentious topic. While it is impossible to cite all relevant research we note that most of the detailed positive evidence for a specific alcohol antagonistic action has involved rats as experimental animals [5, 45, 46], and also the α6R100Q allele was identified using rat lines with different behavioral EtOH sensitivity [7, 38]. The data seem to be much less clear when using mice and this might have contributed to previous controversy whether Ro15-4513 exerts its action via a specific mechanism or if it is simply counteracting alcohol due to its inverse agonist activity on classical γ2-containing synaptic GA-BARs [55]. Indeed, γ2F77I knock-in MICE (the γ2F77I mutation drastically diminishes Ro15-4513 affinity to the majority of classical α+γ− BZ sites), lack Ro15-4513’s alcohol antagonistic effects and this finding supports the view that the modest Ro15-4513’s antagonism of alcohol– induced sedation in WT-MICE is due to Ro15-4513 inverse agonism on classical synaptic GABAR, rather than a specific action on displacing EtOH from EtOH/Ro15-4513 binding sites [56]. In line with this view α4 and α6 global ko MICE did not lead to the much expected reduction in overall EtOH sensitivity, and it is certainly possible that the reported changes in alcohol behavioral measures in δ knock-out MICE [27] are not a result of the removal of the δ subunit, but mostly due to compensatory changes in such global knock-out mouse models. Similarly, in contrast to the α6R100Q mutation in RATS that leads to increased EtOH impairment [8], α6 global knock-out MICE failed to show the much anticipated changes in overall alcohol sensitivity using the loss of righting reflex [57] and also more sensitive assays at EtOH doses as low as 1.5 mg/kg IP for cerebellar motor coordination like rotarod performance [58]. It is fascinating in this regard that mice can become supersensitive to the motor impairing effects of low alcohol doses as observed in the context of potassium channel Kv3.1/Kv 3.3 double ko-out mice, where tonic GABA currents mediated by highly plastic α6βδ GABARs might compensate for the resulting hyper-excitability phenotype [59] .
In summary, there is a lot of evidence for important behavioral alcohol actions mediated by α4/6βδ GABAR in rat models [60, 61]. However, experiments in mice are much less convincing and in some cases argue outright against the notion that δ-GABARs mediate important alcohol actions and Ro15-4513 acts as a specific alcohol antagonist [56]. Therefore, we would like to suggest that at least part of the eternal controversy over the importance of these receptors in behavioral alcohol actions, and whether or not Ro15-4513 is a specific alcohol antagonist, are due to differences in low dose EtOH actions in mice and rats. In fact, we failed to show increased alcohol-induced impairment in α4R100Q knock-in point-mutated mice (Wallner et al., unpublished), that we would have predicted based on the ANT α6R100Q rat mutation.
Another contentious issue has been the failure of others to reproduce EtOH sensitivity of recombinant δ receptors. Our data suggest that this is (at least in part) due to difficulties getting δ subunits to incorporate into functional receptors in recombinant expression systems, even when δ subunits coding mRNA or cDNA are supplied in excess. Consistent with this explanation, α4β3δ receptors expressed in mammalian HEK293 cells show variability in Ro15-4513-sensitive EtOH enhancement, and a functional tagging strategy indicates that this is due to different levels of δ subunit incorporation [62]. In addition, δ subunit expression leads to a rather dramatic increase in the sensitivity to the GABA site ligand THIP, again with variable fractions of receptors that show the THIP sensitivity of native receptors found in neurons in slices [50]. In summary we conclude that the reported failures to mimic the high ethanol (or THIP) sensitivity of tonic currents in recombinant receptor studies [63] is likely due to problems with delta subunit incorporation into recombinant receptors. Approaches with concatenated receptors are difficult to interpret since such concatenated receptor often fail to reflect pharmacological properties of native receptors [48, 64] which indicates that concatenation of subunits to fix the order of subunits in the pentamer might not only result in un-natural positioning of subunits, but also could alter the fragile allosteric conformation that appears to be critical for ligands like THIP and alcohol. Since alcohol/Ro15-4513 binding site on α4/6β3δ receptors proposed here might be utilized to develop novel drugs like alcohol antagonists and also alcohol mimetics (for review see Wallner and Olsen [3] it will be important to develop protocols for reliable recombinant expression of δ-containing receptors that mimic the high THIP and ethanol sensitivity of their native counterparts.
Acknowledgments
This work was supported by a NIH predoctoral fellowship AA015460 to H.J.H., NIH Grants AA017891 and AA021213 to M.W. as well as NIH Grants NS35985, AA007680 and AA021213 to R.W.O.
Abbreviations
- GABAR
GABAA receptors
- BZ
Benzodiazepines
- iBZ
Imidazobenzodiazepine
- AChBP
Acetylcholine binding protein
- THIP
4,5,6,7-tetrahydroisoxazolo(5,4-c)pyridin-3-ol
- Ro15-4513
Ethyl-8-azido-5,6-dihydro-5-methyl-6-oxo-4H-imidazo-1,4-benzodiazepine-3-carboxylate
References
- 1.Liljequist S, Engel J. Effects of GABAergic agonists and antagonists on various ethanol-induced behavioral changes. Psychopharmacol (Berl) 1982;78(1):71–75. doi: 10.1007/BF00470592. [DOI] [PubMed] [Google Scholar]
- 2.Bonetti EP, Burkhard WP, Gabl M, Moöhler H. A partial inverse benzodiazepine agonist Ro15-4513 antagonizes acute ethanol effects in mice and rats. Br J Pharmacol. 1985;86:463. [Google Scholar]
- 3.Wallner M, Olsen RW. Physiology and pharmacology of alcohol: the imidazobenzodiazepine alcohol antagonist site on subtypes of GABAA receptors as an opportunity for drug development? Br J Pharmacol. 2008;154(2):288–298. doi: 10.1038/bjp.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzdak PD, Schwartz RD, Skolnick P, Paul SM. Ethanol stimulates gamma-aminobutyric acid receptor-mediated chloride transport in rat brain synaptoneurosomes. Proc Natl Acad Sci USA. 1986;83(11):4071–4075. doi: 10.1073/pnas.83.11.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzdak PD, Glowa JR, Crawley JN, Schwartz RD, Skolnick P, Paul SM. A selective imidazobenzodiazepine antagonist of ethanol in the rat. Science. 1986;234(4781):1243–1247. doi: 10.1126/science.3022383. [DOI] [PubMed] [Google Scholar]
- 6.Uusi-Oukari M, Korpi ER. Diazepam sensitivity of the binding of an imidazobenzodiazepine, [3H]Ro 15-4513, in cere-bellar membranes from two rat lines developed for high and low alcohol sensitivity. J Neurochem. 1990;54(6):1980–1987. doi: 10.1111/j.1471-4159.1990.tb04901.x. [DOI] [PubMed] [Google Scholar]
- 7.Korpi ER, Kleingoor C, Kettenmann H, Seeburg PH. Benzodiazepine-induced motor impairment linked to point mutation in cerebellar GABAA receptor. Nature. 1993;361(6410):356–359. doi: 10.1038/361356a0. [DOI] [PubMed] [Google Scholar]
- 8.Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M. Alcohol-induced motor impairment caused by increased extra-synaptic GABAA receptor activity. Nat Neurosci. 2005;8(3):339–345. doi: 10.1038/nn1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benson JA, Low K, Keist R, Moöhler H, Rudolph U. Pharmacology of recombinant GABAA receptors rendered diazepam-insensitive by point-mutated α-subunits. FEBS Lett. 1998;431(3):400–404. doi: 10.1016/s0014-5793(98)00803-5. [DOI] [PubMed] [Google Scholar]
- 10.Rudolph U, Crestani F, Moöhler H. GABAA receptor sub-types: dissecting their pharmacological functions. Trends Pharmacol Sci. 2001;22(4):188–194. doi: 10.1016/s0165-6147(00)01646-1. [DOI] [PubMed] [Google Scholar]
- 11.Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL. Sites of alcohol and volatile anaesthetic action on GABAA and glycine receptors. Nature. 1997;389(6649):385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- 12.Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. Hormonally regulated α4β2δ GABAA receptors are a target for alcohol. Nat Neurosci. 2002;5(8):721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances a4b3d and α6β3δ GABAA receptors at low concentrations known to affect humans. Proc Natl Acad Sci USA. 2003;100(25):15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carta M, Mameli M, Valenzuela CF. Alcohol enhances GABA ergic transmission to cerebellar granule cells via an increase in Golgi cell excitability. J Neurosci. 2004;24(15):3746–3751. doi: 10.1523/JNEUROSCI.0067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleming RL, Wilson WA, Swartzwelder HS. Magnitude and ethanol sensitivity of tonic GABAA receptor-mediated inhibition in dentate gyrus changes from adolescence to adulthood. J Neurophysiol. 2007;97(5):3806–3811. doi: 10.1152/jn.00101.2007. [DOI] [PubMed] [Google Scholar]
- 16.Jia F, Pignataro L, Harrison NL. GABAA receptors in the thalamus: α4 subunit expression and alcohol sensitivity. Alcohol. 2007;41(3):177–185. doi: 10.1016/j.alcohol.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Jia F, Chandra D, Homanics GE, Harrison NL. Ethanol modulates synaptic and extrasynaptic GABAA receptors in the thalamus. J Pharmacol Exp Ther. 2008;326(2):475–482. doi: 10.1124/jpet.108.139303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang J, Zhang N, Cagetti E, Houser CR, Olsen RW, Spigelman I. Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J Neurosci. 2006;26(6):1749–1758. doi: 10.1523/JNEUROSCI.4702-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santhakumar V, Wallner M, Otis T. Ethanol acts directly on extrasynaptic subtypes of GABAA receptors to increase tonic inhibition. Alcohol. 2007;41(3):211–221. doi: 10.1016/j.alcohol.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santhakumar V, Meera P, Karakossian MH, Otis TS. A reinforcing circuit action of extrasynaptic GABAA receptor modulators on cerebellar granule cell inhibition. PLoS ONE. 2013;8(8):e72976. doi: 10.1371/journal.pone.0072976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by δ subunit-containing GABAA receptors in hippocampal neurons. J Neurosci. 2004;24(38):8379–8382. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2003;63(1):53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- 23.Liang J, Suryanarayanan A, Abriam A, Snyder B, Olsen RW, Spigelman I. Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication. J Neurosci. 2007;27(45):12367–12377. doi: 10.1523/JNEUROSCI.2786-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen Y, Lindemeyer AK, Spigelman I, Sieghart W, Olsen RW, Liang J. Plasticity of GABAA receptors after ethanol pre-exposure in cultured hippocampal neurons. Mol Pharmacol. 2011;79(3):432–442. doi: 10.1124/mol.110.068650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olsen RW, Liang J, Cagetti E, Spigelman I. Plasticity of GABAA receptors in brains of rats treated with chronic intermittent ethanol. Neurochem Res. 2005;30(12):1579–1588. doi: 10.1007/s11064-005-8836-6. [DOI] [PubMed] [Google Scholar]
- 26.Chandra D, Werner DF, Liang J, Suryanarayanan A, Harrison NL, Spigelman I, Olsen RW, Homanics GE. Normal acute behavioral responses to moderate/high dose ethanol in GABAA receptor α4 subunit knockout mice. Alcohol Clin Exp Res. 2008;32(1):10–18. doi: 10.1111/j.1530-0277.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mihalek RM, Bowers BJ, Wehner JM, Kralic JE, VanDoren MJ, Morrow AL, Homanics GE. GABAA receptor δ subunit knockout mice have multiple defects in behavioral responses to ethanol. Alcohol Clin Exp Res. 2001;25(12):1708–1718. [PubMed] [Google Scholar]
- 28.Teissere JA, Czajkowski C. A β-strand in the γ2 subunit lines the benzodiazepine binding site of the GABAA receptor: structural rearrangements detected during channel gating. J Neurosci. 2001;21(14):4977–4986. doi: 10.1523/JNEUROSCI.21-14-04977.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kucken AM, Teissere JA, Seffinga-Clark J, Wagner DA, Czajkowski C. Structural requirements for imidazobenzodiazepine binding to GABAA receptors. Mol Pharmacol. 2003;63(2):289–296. doi: 10.1124/mol.63.2.289. [DOI] [PubMed] [Google Scholar]
- 30.Horton RM, Ho SN, Pullen JK, Hunt HD, Cai Z, Pease LR. Gene splicing by overlap extension. Methods Enzymol. 1993;217:270–279. doi: 10.1016/0076-6879(93)17067-f. [DOI] [PubMed] [Google Scholar]
- 31.Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411(6835):269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- 32.Schwede T, Kopp J, Guex N, Peitsch MC. SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res. 2003;31(13):3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kucken AM, Wagner DA, Ward PR, Teissere JA, Boileau AJ, Czajkowski C. Identification of benzodiazepine binding site residues in the γ2 subunit of the GABAA receptor. Mol Pharmacol. 2000;57(5):932–939. [PubMed] [Google Scholar]
- 34.Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Moöhler H. Benzodiazepine actions mediated by specific GABAA receptor subtypes. Nature. 1999;401(6755):796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 35.Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, Fritschy JM, Rulicke T, Bluethmann H, Moöhler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290(5489):131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- 36.Carr LG, Spence JP, Peter Eriksson CJ, Lumeng L, Li TK. AA and ANA rats exhibit the R100Q mutation in the GABAA receptor α6 subunit. Alcohol. 2003;31(1–2):93–97. doi: 10.1016/j.alcohol.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Sanna A, Congeddu E, Porcella A, Saba L, Pistis M, Peis M, Marchese G, Ruiu S, Lobina C, Grayson DR, Gessa GL, Pani L. Characterization of wild-type (R100R) and mutated (Q100Q) GABAA α6 subunit in Sardinian alcohol non-preferring rats (sNP) Brain Res. 2003;967(1–2):98–105. doi: 10.1016/s0006-8993(02)04230-0. [DOI] [PubMed] [Google Scholar]
- 38.Uusi-Oukari M, Korpi ER. Cerebellar GABAA receptor binding and function in vitro in two rat lines developed for high and low alcohol sensitivity. Neurochem Res. 1989;14(8):733–739. doi: 10.1007/BF00964950. [DOI] [PubMed] [Google Scholar]
- 39.Olsen RW, Li GD, Wallner M, Trudell JR, Bertaccini EJ, Lindahl E, Miller KW, Alkana RL, Davies DL. Structural models of ligand-gated ion channels: sites of action for anesthetics and ethanol. Alcohol Clin Exp Res. 2013 doi: 10.1111/acer.12283. E-Pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sawyer GW, Chiara DC, Olsen RW, Cohen JB. Identification of the bovine GABAA receptor a subunit residues photolabeled by the imidazobenzodiazepine [3H]Ro15-4513. J Biol Chem. 2002;277(51):50036–50045. doi: 10.1074/jbc.M209281200. [DOI] [PubMed] [Google Scholar]
- 41.Berezhnoy D, Nyfeler Y, Gonthier A, Schwob H, Goeldner M, Sigel E. On the benzodiazepine binding pocket in GABAA receptors. J Biol Chem. 2004;279(5):3160–3168. doi: 10.1074/jbc.M311371200. [DOI] [PubMed] [Google Scholar]
- 42.Hanchar HJ, Chutsrinopkun P, Meera P, Supavilai P, Sieghart W, Wallner M, Olsen RW. Ethanol potently and competitively inhibits binding of the alcohol antagonist Ro15-4513 to α4/6β3δ GABAA receptors. Proc Natl Acad Sci USA. 2006;103(22):8546–8550. doi: 10.1073/pnas.0509903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallner M, Hanchar HJ, Olsen RW. Low dose alcohol actions on α4β3δ GABAA receptors are reversed by the behavioral alcohol antagonist Ro15-4513. Proc Natl Acad Sci USA. 2006;103(22):8540–8545. doi: 10.1073/pnas.0600194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.June HL, Hughes RW, Spurlock HL, Lewis MJ. Ethanol self-administration in freely feeding and drinking rats: effects of Ro15-4513 alone, and in combination with Ro15-1788 (flu-mazenil) Psychopharmacol (Berl) 1994;115(3):332–339. doi: 10.1007/BF02245074. [DOI] [PubMed] [Google Scholar]
- 45.Cook JB, Foster KL, Eiler WJ, 2nd, McKay PF, Woods J, 2nd, Harvey SC, Garcia M, Grey C, McCane S, Mason D, Cummings R, Li X, Cook JM, June HL. Selective GABAA a5 benzodiazepine inverse agonist antagonizes the neurobehavioral actions of alcohol. Alcohol Clin Exp Res. 2005;29(8):1390–1401. doi: 10.1097/01.alc.0000175073.94575.86. [DOI] [PubMed] [Google Scholar]
- 46.McKay PF, Foster KL, Mason D, Cummings R, Garcia M, Williams LS, Grey C, McCane S, He X, Cook JM, June HL. A high affinity ligand for GABAA-receptor containing α5 subunit antagonizes ethanol’s neurobehavioral effects in Long-Evans rats. Psychopharmacol (Berl) 2004;172(4):455–462. doi: 10.1007/s00213-003-1671-z. [DOI] [PubMed] [Google Scholar]
- 47.Mihic SJ, Whiting PJ, Harris RA. Anaesthetic concentrations of alcohols potentiate GABAA receptor-mediated currents: lack of subunit specificity. Eur J Pharmacol. 1994;268(2):209–214. doi: 10.1016/0922-4106(94)90190-2. [DOI] [PubMed] [Google Scholar]
- 48.Kaur KH, Baur R, Sigel E. Unanticipated structural and functional properties of δ subunit-containing GABAA receptors. J Biol Chem. 2009;284(12):7889–7896. doi: 10.1074/jbc.M806484200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagoner KR, Czajkowski C. Stoichiometry of expressed α4β2δ GABAA receptors depends on the ratio of subunit cDNA transfected. J Biol Chem. 2010;285(19):14187–14194. doi: 10.1074/jbc.M110.104257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meera P, Wallner M, Otis TS. Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABAA receptors. J Neurophysiol. 2011;106(4):2057–2064. doi: 10.1152/jn.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mirheydari P, Ramerstorfer J, Varagic Z, Scholze P, Wimmer L, Mihovilovic MM, Sieghart W, Ernst M. Unexpected properties of δ-containing GABA receptors in response to ligands interacting with the α+β− site. Neurochem Res. 2013 doi: 10.1007/s11064-013-1156-3. E-Pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramerstorfer J, Furtmuller R, Sarto-Jackson I, Varagic Z, Sieghart W, Ernst M. The GABAA receptor α+β− interface: a novel target for subtype selective drugs. J Neurosci. 2011;31(3):870–877. doi: 10.1523/JNEUROSCI.5012-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baur R, Tan KR, Luscher BP, Gonthier A, Goeldner M, Sigel E. Covalent modification of GABAA receptor isoforms by a diazepam analogue provides evidence for a novel benzodiazepine binding site that prevents modulation by these drugs. J Neurochem. 2008;106(6):2353–2363. doi: 10.1111/j.1471-4159.2008.05574.x. [DOI] [PubMed] [Google Scholar]
- 54.Walters RJ, Hadley SH, Morris KD, Amin J. Benzodiazepines act on GABAA receptors via two distinct and separable mechanisms. Nat Neurosci. 2000;3(12):1274–1281. doi: 10.1038/81800. [DOI] [PubMed] [Google Scholar]
- 55.Lister RG, Nutt DJ. Is Ro15-4513 a specific alcohol antagonist? Trends Neurosci. 1987;10(6):223–225. [Google Scholar]
- 56.Linden AM, Schmitt U, Leppa E, Wulff P, Wisden W, Luddens H, Korpi ER. Ro15-4513 antagonizes alcohol-induced sedation in mice through αβγ2-type GABAA Receptors. Front Neurosci. 2011;5:3. doi: 10.3389/fnins.2011.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Homanics GE, Ferguson C, Quinlan JJ, Daggett J, Snyder K, Lagenaur C, Mi ZP, Wang XH, Grayson DR, Firestone LL. Gene knockout of the α6 subunit of the GABAA receptor: lack of effect on responses to ethanol, pentobarbital, and general anesthetics. Mol Pharmacol. 1997;51(4):588–596. doi: 10.1124/mol.51.4.588. [DOI] [PubMed] [Google Scholar]
- 58.Korpi ER, Koikkalainen P, Vekovischeva OY, Makela R, Kleinz R, Uusi-Oukari M, Wisden W. Cerebellar granule-cell-specific GABAA receptors attenuate benzodiazepine-induced ataxia: evidence from α6-subunit-deficient mice. Eur J Neurosci. 1999;11(1):233–240. doi: 10.1046/j.1460-9568.1999.00421.x. [DOI] [PubMed] [Google Scholar]
- 59.Espinosa F, McMahon A, Chan E, Wang S, Ho CS, Heintz N, Joho RH. Alcohol hypersensitivity, increased locomotion, and spontaneous myoclonus in mice lacking the potassium channels Kv3.1 and Kv3.3. J Neurosci. 2001;21(17):6657–6665. doi: 10.1523/JNEUROSCI.21-17-06657.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nie H, Rewal M, Gill TM, Ron D, Janak PH. From the cover: extrasynaptic d-containing GABAA receptors in the nucleus accumbens dorsomedial shell contribute to alcohol intake. Proc Natl Acad Sci USA. 2011;108(11):4459–4464. doi: 10.1073/pnas.1016156108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rewal M, Jurd R, Gill TM, He DY, Ron D, Janak PH. Alpha4-containing GABAA receptors in the nucleus accumbens mediate moderate intake of alcohol. J Neurosci. 2009;29(2):543–549. doi: 10.1523/JNEUROSCI.3199-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meera P, Olsen RW, Otis TS, Wallner M. Alcohol- and alcohol antagonist-sensitive human GABAA receptors: tracking δ subunit incorporation into functional receptors. Mol Pharmacol. 2010;78:918–924. doi: 10.1124/mol.109.062687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borghese CM, Storustovu S, Ebert B, Herd MB, Belelli D, Lambert JJ, Marshall G, Wafford KA, Harris RA. The delta subunit of GABAA receptors does not confer sensitivity to low concentrations of ethanol. J Pharmacol Exp Ther. 2006;316(3):1360–1368. doi: 10.1124/jpet.105.092452. [DOI] [PubMed] [Google Scholar]
- 64.Sigel E, Kaur KH, Luscher BP, Baur R. Use of concatamers to study GABAA receptor architecture and function: application to δ subunit-containing receptors and possible pitfalls. Biochem Soc Trans. 2009;37:1338–1342. doi: 10.1042/BST0371338. Pt 6. [DOI] [PubMed] [Google Scholar]