Abstract
The antigenic similarities and differences between highly purified brain tubulins from lamb, mouse, and chick embryo have been examined using rabbit antisera prepared against each of these tubulins. These antisera are capable of binding 125I-labeled tubulin in homologous or heterologous combinations, demonstrating immunological similarity between the tubulins. However, there are quantitative differences in the maximum amount of binding observed. Differences between the tubulins were further resolved by radioimmunoassays, comparing the ability of each of the tubulins to inhibit the binding of each 125I-labeled tubulin to each antiserum. Competition curves generated for all possible combinations revealed quantitative immunological differences between the tubulins that imply different densities of shared antigenic determinants on all three tubulins and a unique determinant on the chick tubulin molecule.
Full text
PDF

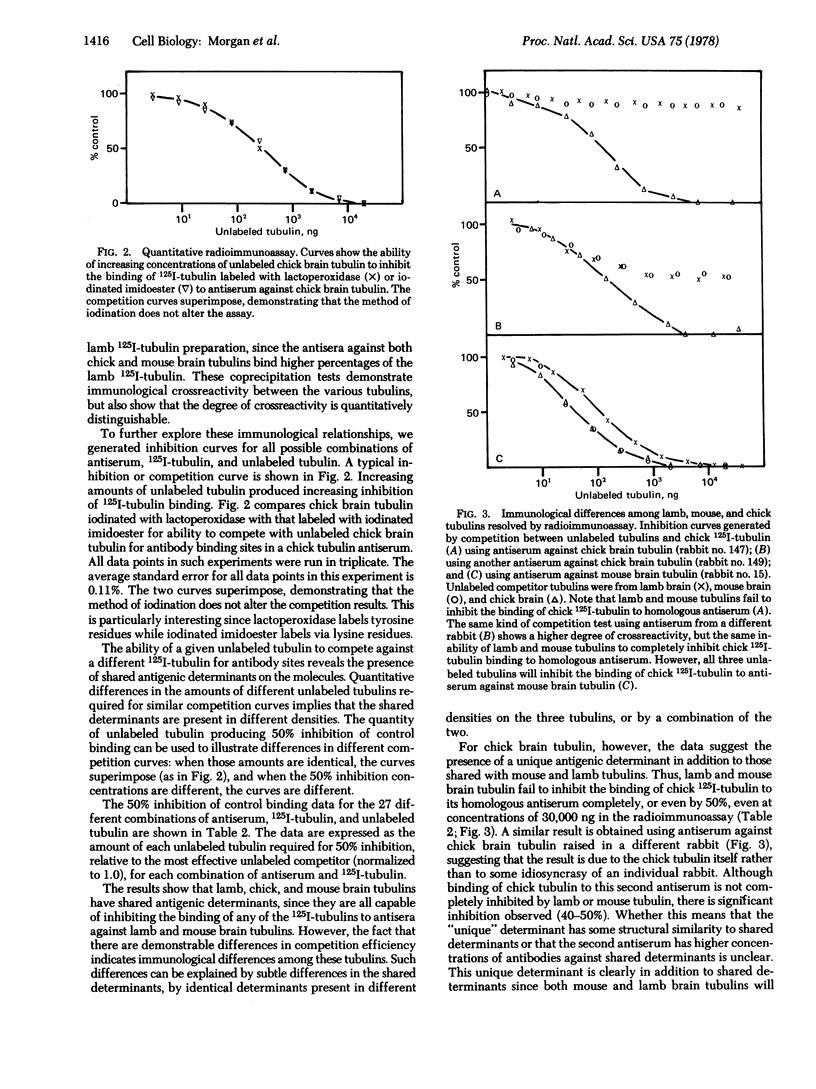

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bibring T., Baxandall J., Denslow S., Walker B. Heterogeneity of the alpha subunit of tubulin and the variability of tubulin within a single organism. J Cell Biol. 1976 May;69(2):301–312. doi: 10.1083/jcb.69.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibring T., Baxandall J. Selective extraction of isolated mitotic apparatus. Evidence that typical microtubule protein is extracted by organic mercurial. J Cell Biol. 1971 Feb;48(2):324–339. doi: 10.1083/jcb.48.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley B. R., Fuller E. M., Highfield D. P. Cytoplasmic microtubules in normal and transformed cells in culture: analysis by tubulin antibody immunofluorescence. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4981–4985. doi: 10.1073/pnas.72.12.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan J., Wilson L. Are cytoplasmic microtubules heteropolymers? Proc Natl Acad Sci U S A. 1971 Aug;68(8):1762–1766. doi: 10.1073/pnas.68.8.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper B. A. Properties of rat brain tubulin. J Biol Chem. 1974 Mar 10;249(5):1407–1416. [PubMed] [Google Scholar]
- Feit H., Slusarek L., Shelanski M. L. Heterogeneity of tubulin subunits. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2028–2031. doi: 10.1073/pnas.68.9.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine R. E. Heterogeneity of tubulin. Nat New Biol. 1971 Oct 27;233(43):283–284. doi: 10.1038/newbio233283a0. [DOI] [PubMed] [Google Scholar]
- Fuller G. M., Brinkley B. R., Boughter J. M. Immunofluorescence of mitotic spindles by using monospecific antibody against bovine brain tubulin. Science. 1975 Mar 14;187(4180):948–950. doi: 10.1126/science.1096300. [DOI] [PubMed] [Google Scholar]
- Fulton C., Kane R. E., Stephens R. E. Serological similarity of flagellar and mitotic microtubules. J Cell Biol. 1971 Sep;50(3):762–773. doi: 10.1083/jcb.50.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himes R. H., Burton P. R., Kersey R. N., Pierson G. B. Brain tubulin polymerization in the absence of "microtubule-associated proteins". Proc Natl Acad Sci U S A. 1976 Dec;73(12):4397–4399. doi: 10.1073/pnas.73.12.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowit J. D., Fulton C. Purification and properties of flagellar outer doublet tubulin from Naegleria gruberi and a radioimmune assay for tubulin. J Biol Chem. 1974 Jun 10;249(11):3638–3646. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Luduena R. F., Woodward D. O. Isolation and partial characterization of alpha and beta-tubulin from outer doublets of sea-urchin sperm and microtubules of chick-embryo brain. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3594–3598. doi: 10.1073/pnas.70.12.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margoliash E., Nisonoff A., Reichlin M. Immunological activity of cytochrome c. I. Precipitating antibodies to monomeric vertebrate cytochromes c. J Biol Chem. 1970 Mar 10;245(5):931–939. [PubMed] [Google Scholar]
- Morgan J. L., Rodkey L. S., Spooner B. S. Quantitation of cytoplasmic tubulin by radioimmunoassay. Science. 1977 Aug 5;197(4303):578–580. doi: 10.1126/science.877574. [DOI] [PubMed] [Google Scholar]
- Murphy D. B., Borisy G. G. Association of high-molecular-weight proteins with microtubules and their role in microtubule assembly in vitro. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2696–2700. doi: 10.1073/pnas.72.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisonoff A., Reichlin M., Margoliash E. Immunological activity of cytochrome c. II. Localization of a major antigenic determinant of human cytochrome c. J Biol Chem. 1970 Mar 10;245(5):940–946. [PubMed] [Google Scholar]
- Olmsted J. B., Witman G. B., Carlson K., Rosenbaum J. L. Comparison of the microtubule proteins of neuroblastoma cells, brain, and Chlamydomonas flagella. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2273–2277. doi: 10.1073/pnas.68.9.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieels J. P., Poortmans J., Dolmans M., Léonis J. Immunological cross-reactions of alpha-lactalbumins from different species. Eur J Biochem. 1975 Jan 15;50(3):523–527. doi: 10.1111/j.1432-1033.1975.tb09892.x. [DOI] [PubMed] [Google Scholar]
- Reichlin M., Nisonoff A., Margoliash E. Immunological activity of cytochrome c. 3. Enhancement of antibody detection and immune response initiation by cytochrome c polymers. J Biol Chem. 1970 Mar 10;245(5):947–954. [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Weber K., Pollack R., Bibring T. Antibody against tuberlin: the specific visualization of cytoplasmic microtubules in tissue culture cells. Proc Natl Acad Sci U S A. 1975 Feb;72(2):459–463. doi: 10.1073/pnas.72.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood F. T., Wu M. M., Gerhart J. C. The radioactive labeling of proteins with an iodinated amidination reagent. Anal Biochem. 1975 Dec;69(2):339–349. doi: 10.1016/0003-2697(75)90136-0. [DOI] [PubMed] [Google Scholar]