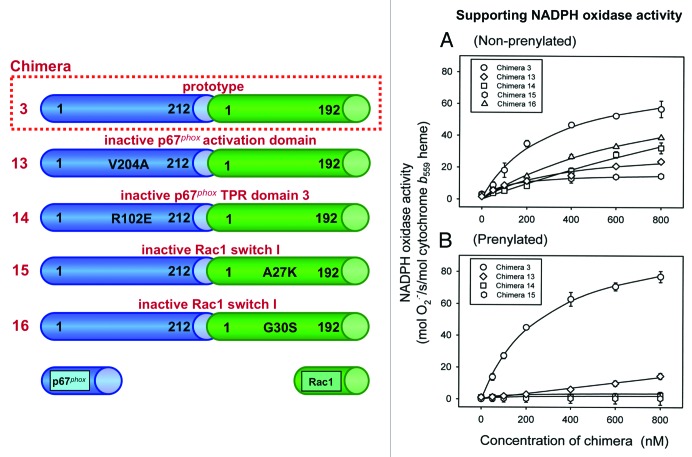
Figure 3. Intrachimeric bonds between residues in the switch I of the Rac1 moiety and in the TPR domains of the p67phox moiety and an intact activation domain in the p67phox moiety of chimera [(p67phox(1–212)-Rac1(1–192)) (prototype chimera 3) are essential for enabling the chimera to support NADPH oxidase activity. The numbering of chimeras are according to Alloul et al.79 Four mutants of the prototype chimera 3 were constructed in which residues in the activation domain and one of the TPR domains of the p67phox moiety and 2 residues in the switch I of the Rac1 moiety were mutated. Graphs A and B describe the ability of mutant chimeras to support NADPH oxidase activation in vitro. (A) NADPH oxidase supporting activity of the mutant chimeras, in non-prenylated form, was assessed in an amphiphile-dependent cell-free assay consisting of phagocyte membrane, chimera in the GTPγS-bound form, and p47phox. (B) The activity of the equivalent prenylated chimeras was measured in an amphiphile-independent cell-free system, consisting of membrane and chimera in the GTPγS-bound form, in the absence of p47phox (modified from ref. 83).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.