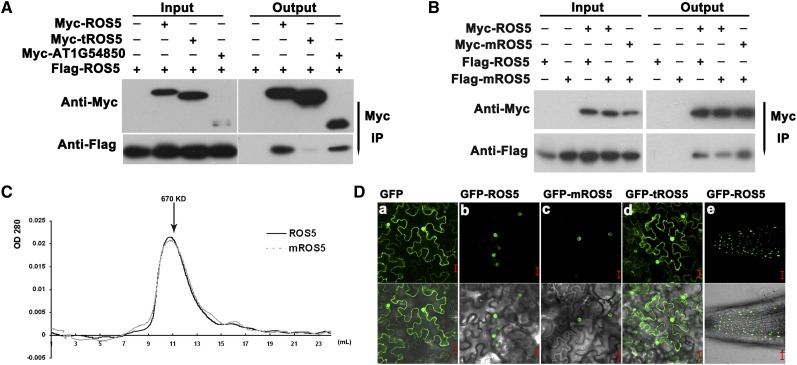
Figure 2.
Characterization of ROS5.
(A) Effect of the ACD on the dimerization of ROS5 as determined by coimmunoprecipitation in a protoplast transient assay. AT1G54850, a close homolog of ROS5, was used as a control. Immunoprecipitation (IP) was conducted with Anti-c-Myc-Agarose Affinity Gel (Sigma-Aldrich).
(B) Effect of the point mutation G246D (mROS5) on ROS5 dimerization as determined by coimmunoprecipitation in a protoplast transient assay. A Myc or Flag tag was fused to the N terminus of ROS5. Samples transformed with only Flag-ROS5 or Flag-mROS5 were used as controls. Immunoprecipitation was conducted with Anti-c-Myc-Agarose Affinity Gel.
(C) Effect of the point mutation G246D on the oligomer formation of ROS5 as determined by gel filtration. Wild-type ROS5 and the point mutation variant mROS5 expressed in Escherichia coli were both assayed at a concentration of 2.0 mg/mL. Protein elution was monitored by A280. Fraction numbers and the relevant molecular mass are shown.
(D) The subcellular localization of GFP-ROS5 (b), GFP-mROS5 (ros5-2) (c), and GFP-tROS5 (ROS5 without ACD) (d) in transient assays in tobacco epidermal cells. Subcellular localization of GFP-ROS5 was also investigated in stable transgenic ros5-1 plants carrying proROS5-GFP-ROS5 (e). GFP itself (a) was used as a control. Bars = 20 μm.