Abstract
Background
Cigarette smoking is an important risk factor for abdominal obesity. However, the degree to which the CYP2A6 genotype moderates the relationship between smoking and abdominal obesity has not been established.
Purpose
This study aims to investigate whether or not the relationship between smoking quantity and abdominal obesity is influenced by CYP2A6 genotypes.
Methods
Nine hundred fifty-four male current smokers were selected. A venous specimen was collected to test serum cotinine and CYP2A6 genotype, and all smokers were divided into heavy (>15 cigarettes/day) and light smokers (≤15 cigarettes/day).
Results
Heavy smoking increased the risk of abdominal obesity (odds ratio (OR)=1.57; 95% CI, 1.13–2.19) compared with light smoking. Furthermore, heavy smoking had a positive interactive effect with CYP2A6 poor metabolizer genotype on abdominal obesity (OR=3.90; 95% CI, 1.25– 12.18). Moreover, CYP2A6 poor metabolizer genotypes were associated with slower nicotine metabolism.
Conclusions
Heavy smoking may increase the risk of abdominal obesity—particularly in smokers with CYP2A6 poor metabolizer genotypes.
Keywords: Cigarette smoking, CYP2A6 genotypes, Abdominal obesity, Interaction
Introduction
Obesity, generally defined as an excess accumulation of body fat [1], is regarded as one of the most important and common public health problems worldwide posing increased risks for type 2 diabetes, many cancers, and heart disease and its risk factors [2, 3]. Cigarette smoking has been associated with body weight and body shape in previous studies. Current smokers tend to have lower body mass index (BMI) but larger waist circumference and waist-to-hip ratios than non-smokers [4–6]. Given that abdominal obesity is a major risk factor for the development of many chronic illnesses and overall mortality [3, 7–9], research exploring the association between abdominal obesity and smoking has major public health implications. Previous studies indicated that waist circumference was more precise than waist-to-hip ratios to assess abdominal obesity [10]. Therefore, in the present study, we chose waist circumference to assess abdominal obesity and further investigated the effect of smoking on waist circumference.
Previous studies suggest that the relationship between smoking and abdominal obesity may be explained by increased plasma levels of nicotine, rather than other constituents of cigarette smoke [6, 11, 12]. Nicotine may increase plasma cortisol levels [13], which in turn could contribute to increased accumulation of abdominal fat [12]. Moreover, it is well established that approximately 70% to 80% of inhaled nicotine is metabolized to cotinine (an inactive metabolite) mainly by CYP2A6 enzyme [14] and that the activity of CYP2A6 enzyme is moderated by variation in the CYP2A6 gene [15, 16]. However, it is unclear whether cigarette smoking and CYP2A6 genotypes have an interactive effect on abdominal obesity.
Therefore, the present study aimed to assess whether or not CYP2A6 genotypes interact with amount of daily cigarette consumption to affect abdominal obesity in Chinese male current smokers.
Methods
Study Subjects
Subjects were from a community-based chronic disease screening project conducted in Guangzhou and Zhuhai of China from July 2006 to June 2007 [17]. In that project, a total of 7,293 residents (2,465 males and 4,828 females) aged 20 years or over were randomly selected using a stratified multistage sampling method. In this population, 1,440 participants were smokers (1,059 current smokers and 381 former smokers or 1,327 male smokers and 113 female smokers). The present study focused on interactions between smoking and CYP2A6 metabolizer status on abdominal obesity. Therefore, given that there were so few female smokers in the group, thereby limiting statistical power, the genotyped study population was limited to the 1,025 male current smokers in the cohort. Of the 1,025 current male smokers, however, 71 refused to provide blood samples resulting in a final study sample of 954 male current smokers. Figure 1 provides additional details on the selection of the study sample. This study was approved by the Ethics Committees of Sun Yat-sen University in Guangzhou, China, and written informed consent was obtained from all participants.
Fig. 1. Study participants selection diagram.
Data Collection
All the sampled subjects were interviewed with a structured questionnaire by trained medical students or clinical doctors inquiring about their socio-demographic characteristics, smoking behaviors, consumption of alcohol, caffeine, diet, and physical activity [17]. Anthropometric indices including height, weight, and waist circumference were also measured and blood samples were collected via venipuncture. All clinical evaluations and data collection were conducted at local health care centers, and methods for data collection are described in detail elsewhere [17].
Measurement and Definition of Cigarette Smoking and Other Lifestyle Factors
A “current smoker” was defined by having smoked greater than 100 cigarettes in one's lifetime and having smoked at least one cigarette daily at the time of the interview [18]. Daily average cigarette consumption was reported for current smokers. Alcohol, tea and coffee consumption were divided into two categories (ever or never) based on whether a subject had at any time consumed alcohol, tea or coffee at least 3 times a week for more than 6 months [19]. Physical activity was also classified into two categories: regular physical activity defined as leisure time physical activity engaged in any intensity for 30 min at least three times a week, otherwise defined as no regular physical activity group [20].
Measurement of Obesity and Abdominal Obesity
Body height was measured to the nearest centimeter. Body weight was measured to the nearest 0.1 kg using a digital bathroom scale while the subjects were barefoot and wearing light clothing. BMI was calculated as weight (in kg) divided by the square of height (in m). Participants with a BMI of ≥23 and <30 kg/m2 were classified as overweight, and those with a BMI of ≥30 kg/m2 were classified as obese [21]. Waist circumference at the navel level and hip circumference were measured in duplicate with the subjects standing and at the end of expiration under normal breathing, and the average value was used in the present study. Abdominal obesity was defined by waist circumference of ≥85 cm [22].
CYP2A6 Genotyping
The selection of CYP2A6 alleles assayed in the present study was based on two factors: (a) the impact of the genetic variant on CYP2A6 enzyme function and (b) the frequency of the variant in Chinese populations. The genotyping of CYP2A6*4 [23], CYP2A6*9 [24], CYP2A6*5 [25], CYP2A6*7, and CYP2A6*10 [26] was performed, with minor modifications to the CYP2A6*5 assay and with the first and second amplification primers changed to the R6 [27] and R0 [28] as we have previously described [29]. The 954 current male smokers were divided into four groups (normal, intermediate, slow, and poor CYP2A6 metabolizer genotypes) based on the predicted pharmacokinetic impact of genotypes resulting from the different variant alleles studied [29].
Measurement of Serum Cotinine Concentration
Serum cotinine (the main metabolite of nicotine with a half-life of 16 h) concentrations were measured in the 954 current male smokers rather than nicotine directly because of the relatively shorter half-life of nicotine (1–2 h) compared to cotinine (13–18 h) [14], which makes accurate assessment of individual nicotine levels directly not possible. Given that approximately 70% to 80% of inhaled nicotine is metabolized to cotinine [14], we posited that for a given number of cigarettes smoked, the higher level of serum cotinine concentrations observed, the lower bioavailability of nicotine. Therefore, it was further assumed that smokers with higher levels of serum cotinine would have a higher BMI and/or lower waist circumfer-ence when their daily cigarettes consumption was controlled. The serum cotinine was tested by enzyme-linked immunosorbent assay kit, provided by Immunalysis Corporation, Pomona, CA, USA. The technique is sensitive to within ±1 ng/ml.
Statistical Analysis
For continuous variables, means ± standard deviation were calculated. Categorical variables were given as percentage of subjects with the respective attribute. Several Chi-square tests were performed to test the differences between the baseline characteristics of male current smokers with and without blood samples.
A series of binary logistic regression models was used to analyze the binary relationships between amounts of daily cigarette consumption (0 = 1–15 (light smokers) cigarettes/day, 1= larger than 15 cigarettes/day (heavy smokers)) defined by the median of cigarettes per day (i.e., 15), serum cotinine (0<225.31 and 1≥225.31 ng/ml) defined by the median of cotinine level (i.e., 225.31 ng/ml), CYP2A6 genotypes (0=normal metabolizers, 1 = intermediate metab-olizers, 2=slow metabolizers, and 3=poor metabolizers) and abdominal obesity in 954 current male smokers with blood samples.
Daily cigarette consumption and CYP2A6 genotypes measures were first entered into a binary logistic regression model of abdominal obesity defined by waist circumference, then an interaction term between amounts of daily cigarette consumption and CYP2A6 genotypes was further added into the model. Current smokers were divided into eight groups, stratified by heavy/light smoking and CYP2A6 genotype with light smokers possessing normal metabolizer genotypes as the reference group. The effect sizes for the comparisons of risk for abdominal obesity between these groups were assessed by a binary logistic regression. A three-dimensional bar graph of the eight corresponding odds ratios (OR) illustrate these comparisons (Fig. 2). In these logistic regression models, all independent variables were introduced using the “enter method.”
Fig. 2.
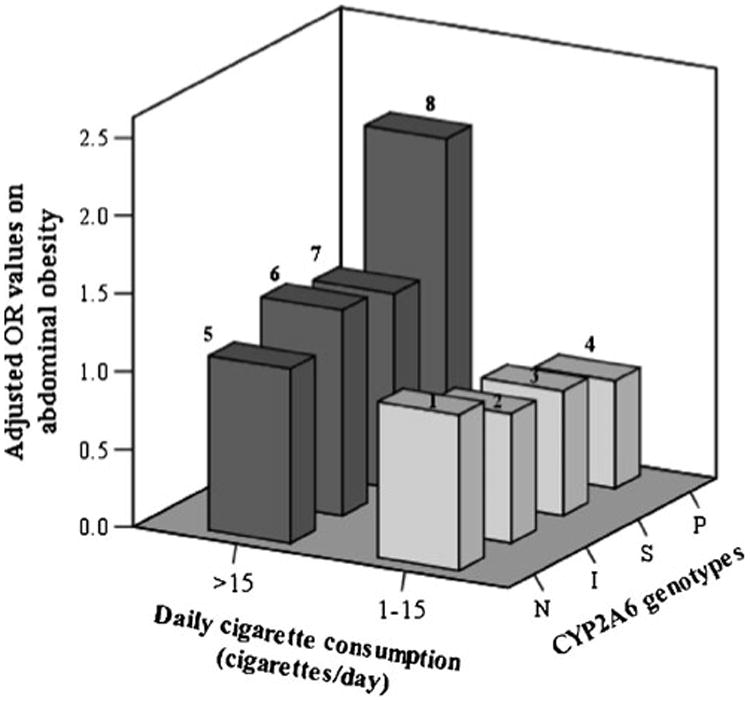
Effect sizes (adjusted ORs) of amounts of daily cigarette consumption and CYP2A6 genotypes on abdominal obesity in eight groups stratified by both daily cigarette consumption and CYP2A6 genotypes in 954 current male smokers with blood samples. The group that consumed 1–15 cigarettes/day and CYP2A6 normal metabolizer genotype was the reference group (OR=1). The adjusted variables were age, occupation, education, family income, alcohol consumption, exercise, coffee consumption, tea consumption, and BMI. The number of smokers in the eight groups were 243 (group 1), 73 (group 2), 146 (group 3), 83 (group 4), 198 (group 5), 64 (group 6), 108 (group 7), and 39 (group 8), respectively. N normal CYP2A6 metabolizer genotype, I intermediate CYP2A6 metabolizer genotype, S slow CYP2A6 metabolizer genotype, P poor CYP2A6 metabolizer genotype
Potential confounding factors were adjusted for in the analysis, including age, occupation, family monthly income, alcohol consumption, exercise, coffee consumption, tea consumption, and BMI. Confounding factors were defined as the factors that explain or produce all or part of the difference between the measure of association and the measure of effect that would be obtained with a counterfactual ideal [30].
The frequencies of CYP2A6*4, CYP2A6*5, CYP2A6*7, CYP2A6*9, and CYP2A6*10 alleles were in Hardy–Weinberg distribution (p>0.05) and were of similar frequency to those previously observed in Chinese samples [29]. All p values were two-sided (α=0.05). All analyses were conducted with SPSS 13.0 software (SPSS, Inc., Chicago, IL USA).
Results
Comparison of Characteristics Between Male Current Smokers with and Without Blood Samples
There were significant differences of age, occupation, family history of hypertension, exercise, BMI, and abdominal obesity distribution between the two groups of subjects. More details are presented in Table 1.
Table 1. Comparisons of characteristics of 1,025 male current smokers with and without blood samples.
| Variables | Current smokers without blood samples (n=71) | Current smokers with blood samples (n=954) | χ2 | p value | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| N | % | N | % | |||
| 20∼29 | 9 | 12.7 | 38 | 4.0 | 52.10 | <0.001 |
| 30∼39 | 25 | 35.2 | 108 | 11.3 | ||
| 40∼49 | 9 | 12.7 | 203 | 21.3 | ||
| 50∼59 | 17 | 23.9 | 325 | 34.1 | ||
| 60∼69 | 5 | 7.0 | 216 | 22.6 | ||
| 70∼86 | 6 | 8.5 | 64 | 6.7 | ||
| Occupation | ||||||
| Worker | 18 | 25.4 | 235 | 24.6 | 16.31 | 0.022 |
| Farmer | 13 | 18.3 | 115 | 12.0 | ||
| Person in charge | 1 | 1.4 | 52 | 5.5 | ||
| Technician | 6 | 8.5 | 43 | 4.5 | ||
| Service personnel | 16 | 22.5 | 120 | 12.6 | ||
| Retired personnel | 9 | 12.7 | 222 | 23.3 | ||
| Jobless | 5 | 7.0 | 121 | 12.7 | ||
| Others | 3 | 4.2 | 46 | 4.8 | ||
| Education | ||||||
| Illiteracy | 2 | 2.8 | 21 | 2.2 | 5.52 | 0.238 |
| Elementary school | 17 | 23.9 | 149 | 15.6 | ||
| Junior middle school | 18 | 25.4 | 338 | 35.4 | ||
| Senior middle school or vocational secondary school | 22 | 31.0 | 317 | 33.3 | ||
| College or above | 12 | 16.9 | 129 | 13.5 | ||
| Family monthly income (yuan) | ||||||
| <1,000 | 13 | 18.3 | 128 | 13.4 | 1.98 | 0.739 |
| 1,000∼2,999 | 20 | 28.2 | 317 | 33.2 | ||
| 3,000∼4,999 | 18 | 25.4 | 262 | 27.5 | ||
| ≥5,000 | 13 | 18.3 | 153 | 16.0 | ||
| Don’t know or refuse to answer | 7 | 9.8 | 94 | 9.9 | ||
| Family history of hypertension | ||||||
| No | 58 | 81.7 | 665 | 69.7 | 4.57 | 0.033 |
| Yes | 13 | 18.3 | 289 | 30.3 | ||
| Alcohol consumption | ||||||
| No | 51 | 71.8 | 603 | 63.2 | 2.41 | 0.300 |
| Yes | 18 | 25.4 | 299 | 31.3 | ||
| Former drinker | 2 | 2.8 | 52 | 5.5 | ||
| Exercise | ||||||
| No | 38 | 53.5 | 366 | 38.4 | 6.36 | 0.012 |
| Yes | 33 | 46.5 | 588 | 61.6 | ||
| Obesity | ||||||
| Normal | 43 | 60.6 | 422 | 44.2 | 8.17 | 0.017 |
| Overweight | 28 | 39.4 | 506 | 53.0 | ||
| Obese | 0 | 0.0 | 26 | 2.8 | ||
| Abdominal obesity | ||||||
| No | 54 | 76.1 | 581 | 60.9 | 6.44 | 0.011 |
| Yes | 17 | 23.9 | 373 | 39.1 | ||
Association among Smoking Quantity, Serum Cotinine, CYP2A6 Genotypes, and Abdominal Obesity in Current Smokers
Table 2 presents the results of associations among smoking quantity, serum cotinine, CYP2A6 genotypes, and abdominal obesity in male current smokers (n=954) after adjusting for potential confounders. Heavy smokers had a higher risk of abdominal obesity than light smokers (OR=1.57; 95% CI, 1.13–2.19). Smokers with higher levels of serum cotinine had a significant lower waist circumference (OR=0.61; 95% CI, 0.41–0.90) and smoked significantly more cigarettes than smokers with lower serum cotinine. Smokers with CYP2A6 poor metabolizer genotype smoked fewer cigarettes per day (OR=0.59; 95% CI, 0.38–0.90) and had lower levels of serum cotinine (OR=0.52; 95% CI, 0.34–0.79) than smokers with CYP2A6 normal metabolizer genotype.
Table 2. The associations between amounts of daily cigarette consumption, serum cotinine, CYP2A6 genotypes, and abdominal obesity in 954 current male smokers with blood samples.
| Number (%) | Abdominal obesity | Amounts of daily cigarette consumption (>15 cigarettes/day) | Serum cotinine (≥225.31 ng/ml) | |
|---|---|---|---|---|
| Adjusted OR (95% CI) | Adjusted OR (95% CI) | Adjusted OR (95% CI) | ||
| Amounts of daily cigarette consumption | ||||
| 1–15 cigarettes/day | 545 (57.1) | 1 | ||
| >15 cigarettes/day | 409 (42.9) | 1.57* (1.13–2.19)a | ||
| Serum cotinine | ||||
| <225.31 ng/ml | 477 (50.0) | 1 | 1 | |
| ≥225.31 ng/ml | 477 (50.0) | 0.61* (0.41–0.90)b | 2.53* (1.92–3.32)c | |
| CYP2A6 genotypes | ||||
| CYP2A6 normal metabolizer genotype | 441 (46.2) | 1 | 1 | 1 |
| CYP2A6 intermediate metabolizer genotype | 137 (14.4) | 0.72 (0.41–1.26)a | 1.03 (0.70–1.53)c | 1.08 (0.73–1.60)c |
| CYP2A6 slow metabolizer genotype | 254 (26.6) | 0.90 (0.57–1.41)a | 0.89 (0.65–1.22)c | 0.89 (0.65–1.22)c |
| CYP2A6 poor metabolizer genotype | 122 (12.8) | 0.94 (0.53–1.66)a | 0.59* (0.38–0.90)c | 0.52* (0.34–0.79)c |
p<0.05
Binary logistic regression adjusted for age, occupation, education, family income, alcohol consumption, exercise, coffee consumption, tea consumption, and BMI
Binary logistic regression adjusted for age, occupation, education, family income, alcohol consumption, exercise, coffee consumption, tea consumption, daily cigarettes consumption, and BMI
Binary logistic regression adjusted for age, occupation, education, family income, alcohol consumption, exercise, coffee consumption, and tea consumption
Interaction Between Cigarette Smoking and CYP2A6 Genotypes on Abdominal Obesity
After adjustment for potential confounding factors, there was an interaction between heavy smoking and CYP2A6 genotype on abdominal obesity such that individuals with CYP2A6 poor metabolizer genotypes were more likely to have abdominal obesity if they were heavy smokers compared with light smokers (ORCYP2A6 poor metabolizer genotype × heavy smoking=3.90; 95% CI, 1.25–12.18) (Table 3). Moreover, although not statistically significant, compared with the light smokers with normal CYP2A6 metabolizer genotypes, heavy smokers with poor CYP2A6 metabolizer genotype trended towards a higher risk of abdominal obesity (OR=2.07; 95% CI, 0.85–5.01) (Fig. 2).
Table 3. The interaction effect between amounts of daily cigarette consumption and CYP2A6 genotypes on abdominal obesity in 954 current male smokers.
| Abdominal obesit | Main effect of amounts of daily cigarette consumption | Main effect of CYP2A6 genotypes | Interaction effect | ||
|---|---|---|---|---|---|
|
|
|
|
|
||
| Controls (N (%)) | Cases (N (%)) | Model 1 Adjusted OR (95% CI) | Model 2 Adjusted OR (95% CI) | Model 3 Adjusted OR (95% CI) | |
| Waist circumference (abdominal obesity)a | |||||
| Daily cigarettes consumption | |||||
| 1–15 cigarettes/day | 358 (61.6) | 187 (50.1) | 1 | 1 | |
| >15 cigarettes/day | 223 (38.4) | 186 (49.9) | 1.57* (1.13–2.19)b | 1.21 (0.69–2.10)b | |
| CYP2A6 genotype | |||||
| NM | 270 (46.5) | 171 (45.8) | 1 | 1 | |
| IM | 91 (15.7) | 46 (12.3) | 0.72 (0.41–1.26)b | 0.36* (0.16–0.81)b | |
| SM | 147 (25.3) | 107 (28.7) | 0.90 (0.57–1.41)b | 0.74 (0.41–1.37)b | |
| PM | 73 (12.5) | 49 (13.2) | 0.94 (0.53–1.66)b | 1.21 (0.69–2.10)b | |
| Interaction effect | |||||
| IM×(>15 cigarettes/day) | 1.50 (0.60–3.74)b | ||||
| SM×(>15 cigarettes/day) | 2.36 (0.67–8.31)b | ||||
| PM×(>15 cigarettes/day) | 3.90* (1.25–12.18)b | ||||
NM CYP2A6 normal metabolizer genotype, IM CYP2A6 intermediate metabolizer genotype, SM CYP2A6 slow metabolizer genotype, PM CYP2A6 poor metabolizer genotype
p<0.05
Dependent variable in the binary logistic regression model (0=normal and 1=abdominal obesity)
OR value adjustment for age, occupation, education, family income, alcohol consumption, exercise, coffee consumption, tea consumption, and BMI
Discussion
To our knowledge, this is the first study to report analyses of joint associations between daily cigarette consumption and CYP2A6 genotypes with abdominal obesity. In this cross-sectional study, it was found that heavy smokers, as indicated by consuming >15 cigarettes/day, had a larger waist circumference compared with light smokers. After adjustment for amount of daily cigarette consumption and other potential confounding factors, serum cotinine was negatively correlated to waist circumference. More importantly, heavy cigarette smoking interacted with CYP2A6 genotypes on abdominal obesity as defined by waist circumference, suggesting that subjects with poor metabo-lizer CYP2A6 genotypes who are heavy smokers are a high risk group for abdominal obesity when compared with light smokers with normal metabolizer CYP2A6 genotypes.
The Effect of Cigarette Smoking on Abdominal Obesity
It is well documented that smoking may increase the risk of abdominal obesity. For example, Rose and colleagues observed that current cigarette smoking was associated with greater central adiposity [31]. Moreover, several studies have reported that current smokers tend to have larger waist circumference or waist-to-hip ratios than never smokers [5, 6, 32–35], and a study by Mizuno and colleagues found that obese smokers had a larger waist circumference than obese non-smokers and that there was no difference in waist circumference between smokers and non-smoker in non-obese subjects [36]. Similarly, the present study observed that heavy smokers had a larger waist circumference compared with light smokers. Furthermore, the Framingham Heart Study and a study in Japan respectively found that cigarette smoking was associated with higher accumulation of visceral adipose tissue compared to never smokers [37, 38].
The mechanism by which cigarette smoking increases the accumulation of visceral adipose tissue is unclear, but three hypotheses have been proposed. The first proposed mechanism is that smoking's anti-estrogenic effect [11], which is related to a hormonal imbalance, can lead to abdominal obesity [39]. The second hypothesis is that higher levels of nicotine intake may increase plasma cortisol levels [13], and that this elevation of plasma cortisol is associated with abdominal adiposity [12]. The third theory posits that cigarette smoking may also induce a heightened activity of gluteal adipose tissue lipoprotein lipase, resulting in up-regulation of the uptake and storage of triglyceride fatty acids by the abdominal adipocytes and consequent increases in abdominal fat mass [6]. In addition, a higher prevalence of other unhealthy habits (e.g., less physical exercise, higher alcohol consumption, and less consumption of fresh vegetables and fruits) among heavy smokers may also influence the abdominal obesity [12, 40].
Interaction between Cigarette Smoking and CYP2A6 Genotypes on Abdominal Obesity
CYP2A6 is the major enzyme mediating nicotine metabolism which can alter smoking behaviors due to differential nicotine clearance, in addition to its role in activating a number of tobacco-specific nitrosamines including 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), N-nitrosonornicotine, N-nitrosodiethylamine, and polycyclic aromatic hydrocarbons into potentially carcinogenic forms [41]. Most of the functionally important polymorphic alleles of CYP2A6 either result in abolished activity (*2, *4, *5, and *20) or reduced activity (*6, *7, *10, *11, *12, *17, *18, and *19), and CYP2A6 genetic variation is related to smoking behavior [42, 43] and tobacco-related cancer risk [41, 44]. Smokers with more rapid nicotine metabolism are at higher increased risk of lung cancer [45, 46], nasopharyngeal carcinoma [47], pancreatic cancer [48], bladder cancer [49], and head and neck cancer [50] compared with smokers with low rates of nicotine metabolism. A possible mechanistic explanation for these apparent CYP2A6 by smoking interactions on risk of cancer may be that compared to smokers with slower nicotine metabolism, smokers with faster nicotine metabolism smoke more cigarettes and have increased rates of bioactivation of many carcinogens, such as bioactivated NNK, that may contribute to the increased risk of tobacco-related cancers [46].
An interaction between amount of daily cigarette consumption and CYP2A6 genotypes on abdominal obesity was observed in the present study. When controlling for age, occupation, education, family income, exercise, caffeine intake and BMI, heavy smokers with poor metabo-lizer genotypes were at significantly increased risk of abdominal obesity compared with light smokers with the normal metabolizer genotype. As mentioned above, approximately 80% of nicotine is metabolized by CYP2A6 into cotinine via C-oxidation, and cotinine is further metabolized into trans-3hydroxycotinine by the same enzyme, which is subsequently excreted in the urine [41]. Differences in the rate of nicotine metabolism are associated with CYP2A6 enzymatic activity, which is moderated by CYP2A6 genetic polymorphisms [41, 46, 51]. We would therefore expect that when individuals with CYP2A6 poor metabolizer genotypes, relative to those with normal metabolizer genotypes, smoke more than 15 cigarettes per day, they metabolize nicotine into cotinine more slowly, leading to the accumulation of higher levels of nicotine in the body. The relatively higher levels of plasma nicotine in heavy smoking poor metabolizers compared with those who metabolize more quickly could contribute to increased risk for abdominal obesity as described above [12, 13].
Limitations
Some limitations need to be mentioned in the present study. A cross-sectional study design was used in the present study, which limited our ability to infer a causal relationship. Second, 71 male smokers without blood samples were excluded from the present study (they differed on some demographic characteristics from the smokers with blood samples, such as age, occupation, and family history of hypertension), which could alter generalizability. In addition, fat mass and lean mass (such as gluteal muscle mass) were not measured preventing us from assessing the effect of smoking on body composition in smokers. Not all CYP2A6 genetic variants were assayed, which might result in individuals being grouped in faster metabolic groups than they should have (e.g., some in the normal group likely have untested variants); this would tend to reduce statistical power. Cotinine was measured, instead of the more pharmacologically relevant nicotine, due to the fast and variable rates of nicotine metabolism. Thus, it was not possible to (a) examine nicotine levels directly and (b) to test whether there was an inverse relationship between cotinine and nicotine, for any given level of smoking, and whether this relationship was altered by genotype. The statistical power was insufficient (67.4%) to analyze the effect of smoking quantity (continuous measure) on abdominal obesity. Finally, participants’ dietary and drug histories were not investigated either, both of which might affect the activity of CYP2A6 [14] and could distort the relationship of CYP2A6 genotypes, amounts of daily cigarette consumption and abdominal obesity.
Conclusions
In summary, heavy smoking was significantly positively associated with abdominal obesity, and the association between amounts of daily cigarette consumption and abdominal obesity may be moderated by CYP2A6 genotypes in Chinese male current smokers. These findings extend our understanding of the effect of cigarettes smoking on abdominal obesity.
Acknowledgments
This study was funded by the Guangzhou Health Bureau (2005-Zda-001). National Institute on Drug Abuse/National Institutes of Health grants K08-014276 and R21-027331. We also acknowledge the financial support from the Centre for Addiction and Mental Health (RFT QZ) and Canadian Institutes for Health Research MOP86471. This study was completed with the assistance of the following units: Disease Prevention and Control Center of Haizhu District and Baiyun District, the Health Inspection Institute of Huangpu District, the Sixth Affiliated Hospital of Sun Yat-sen University, and the Health Care Center of Huayin Community, Jinyang Community, Longteng Community, and the Department of Health Education of Liwan Hospital.
Footnotes
Conflicts of interest statement: Dr.Tyndale owns shares and participates in Nicogen Research Inc., a company focused on novel smoking cessation treatment approaches. No Nicogen funds were used in this work, and no other Nicogen participants reviewed the manuscript. Dr. Tyndale has also consulted for Novartis. All other authors have no conflicts of interests to declare.
Contributor Information
Tao Liu, Department of Biostatistics and Epidemiology, School of Public Health, Sun Yat-Sen University, 74, Zhongshan Road 2, Guangzhou, China; Guangdong Provincial Key Laboratory of Food, Nutrition and Health, School of Public Health, Sun Yat-Sen University, 74, Zhongshan Road 2, Guangzhou, China
Sean P. David, Center for Education in Family and Community Medicine, 1215 Welch Road, Modular G, Stanford, CA 94305, USA.
Rachel F. Tyndale, The Center for Addiction and Mental Health and the Departments of Pharmacology and Toxicology and Psychiatry, University of Toronto, Toronto, ON M5S 1A8, Canada
Hui Wang, Department of Biostatistics and Epidemiology, School of Public Health, Sun Yat-Sen University, 74, Zhongshan Road 2, Guangzhou, China; Guangdong Provincial Key Laboratory of Food, Nutrition and Health, School of Public Health, Sun Yat-Sen University, 74, Zhongshan Road 2, Guangzhou, China
Xue-Qing Yu, Department of Nephrology, The First Affiliated Hospital of Sun Yat-Sen University, 58, Zhongshan Road 2, Guangzhou, China
Wei Chen, Department of Nephrology, The First Affiliated Hospital of Sun Yat-Sen University, 58, Zhongshan Road 2, Guangzhou, China
Qian Zhou, The Center for Addiction and Mental Health and the Departments of Pharmacology and Toxicology and Psychiatry, University of Toronto, Toronto, ON M5S 1A8, Canada
Wei-Qing Chen, Email: chenwq@mail.sysu.edu.cn, Department of Biostatistics and Epidemiology, School of Public Health, Sun Yat-Sen University, 74, Zhongshan Road 2, Guangzhou, China; Guangdong Provincial Key Laboratory of Food, Nutrition and Health, School of Public Health, Sun Yat-Sen University, 74, Zhongshan Road 2, Guangzhou, China.
References
- 1.Prentice AM, Jebb SA. Beyond body mass index. Obes Rev. 2001;2:141–147. doi: 10.1046/j.1467-789x.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i, xii, 1–253. [PubMed] [Google Scholar]
- 3.Corrada MM, Kawas CH, Mozaffar F, Paganini-Hill A. Association of body mass index and weight change with all-cause mortality in the elderly. Am J Epidemiol. 2006;163:938–949. doi: 10.1093/aje/kwj114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albanes D, Jones DY, Micozzi MS, Mattson ME. Associations between smoking and body weight in the US population: Analysis of NHANES II. Am J Public Health. 1987;77:439–444. doi: 10.2105/ajph.77.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bamia C, Trichopoulou A, Lenas D, Trichopoulos D. Tobacco smoking in relation to body fat mass and distribution in a general population sample. Int J Obes Relat Metab Disord. 2004;28:1091–1096. doi: 10.1038/sj.ijo.0802697. [DOI] [PubMed] [Google Scholar]
- 6.Canoy D, Wareham N, Luben R, Welch A, Bingham S, Day N, et al. Cigarette smoking and fat distribution in 21,828 British men and women: A population-based study. Obes Res. 2005;13:1466–1475. doi: 10.1038/oby.2005.177. [DOI] [PubMed] [Google Scholar]
- 7.Kannel WB, D'Agostino RB, Cobb JL. Effect of weight on cardiovascular disease. Am J Clin Nutr. 1996;63:419S–422S. doi: 10.1093/ajcn/87.6.1602. [DOI] [PubMed] [Google Scholar]
- 8.Resnick HE, Valsania P, Halter JB, Lin X. Relation of weight gain and weight loss on subsequent diabetes risk in overweight adults. J Epidemiol Community Health. 2000;54:596–602. doi: 10.1136/jech.54.8.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.S. Environmental Protection Agency, Office of Health and Environmental Assessment. Respiratory Health Effects of Passive Smoking Lung Cancer and Other Disorders. EPA/600/6-90/006F. 1992:44–96. [Google Scholar]
- 10.Pasupathi P, Bakthavathsalam G, Rao YY, Farook J. Cigarette smoking—Effect of metabolic health risk: A review. Diabetes & Metabolic Syndrome. 2009;3:120–127. [Google Scholar]
- 11.Tanko LB, Christiansen C. An update on the antiestrogenic effect of smoking: A literature review with implications for researchers and practitioners. Menopause. 2004;11:104–109. doi: 10.1097/01.GME.0000079740.18541.DB. [DOI] [PubMed] [Google Scholar]
- 12.Sulander T, Rahkonen O, Nissinen A, Uutela A. Association of smoking status with obesity and diabetes among elderly people. Arch Gerontol Geriatr. 2007;45:159–167. doi: 10.1016/j.archger.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Gilbert DG, Meliska CJ, Williams CL, Jensen RA. Subjective correlates of cigarette-smoking-induced elevations of peripheral beta-endorphin and cortisol. Psychopharmacology. 1992;106:275–281. doi: 10.1007/BF02801984. [DOI] [PubMed] [Google Scholar]
- 14.Hukkanen J, Jacob P, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 15.Nakajima M, Yokoi T. Interindividual variability in nicotine metabolism: C-oxidation and glucuronidation. Drug Metab Pharmacokinet. 2005;20:227–235. doi: 10.2133/dmpk.20.227. [DOI] [PubMed] [Google Scholar]
- 16.Mwenifumbo JC, Tyndale RF. Genetic variability in CYP2A6 and the pharmacokinetics of nicotine. Pharmacogenomics. 2007;8:1385–1402. doi: 10.2217/14622416.8.10.1385. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Qiu Q, Tan LL, Liu T, Deng XQ, Chen YM, et al. Prevalence and determinants of diabetes and impaired fasting glucose among urban community-dwelling adults in Guangzhou, China. Diabetes Metab. 2009;35:378–384. doi: 10.1016/j.diabet.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control. National Health Interview Survey. [Accessed 1 April 2011];Early release of selected estimates based on data from January to September 2007. Available at www.cdc.gov.
- 19.Wen W, Xiang YB, Zheng W, Xu WH, Yang G, Li H, et al. The association of alcohol, tea, and other modifiable lifestyle factors with myocardial infarction and stroke in Chinese men. CVD Prev Control. 2008;3:133–140. doi: 10.1016/j.cvdpc.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park MS, Chung SY, Chang Y, Kim K. Physical activity and physical fitness as predictors of all-cause mortality in Korean men. J Korean Med Sci. 2009;24:13–9. doi: 10.3346/jkms.2009.24.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO/IASO/IOTF. The Asia-Pacific perspective: Redefining obesity and its treatment. Melbourne: Health Communications Australia; 2000. [Google Scholar]
- 22.Coorperative Meta-analysis Group of China Obesity Task Force. Predictive values of body mass index and waist circumference to risk factors of related diseases in Chinese adult population. Chin J Epidemiol. 2002;23:5–10. [PubMed] [Google Scholar]
- 23.Lerman C, Jepson C, Wileyto EP, Patterson F, Schnoll R, Mroziewicz M, et al. Genetic variation in nicotine metabolism predicts the efficacy of extended-duration transdermal nicotine therapy. Clin Pharmacol Ther. 2010;87:553–557. doi: 10.1038/clpt.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schoedel KA, Hoffmann EB, Rao Y, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics. 2004;14:615–626. doi: 10.1097/00008571-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Oscarson M, McLellan RA, Gullsten H, Agundez JA, Benitez J, Rautio A, et al. Identification and characterisation of novel polymorphisms in the CYP2A locus: Implications for nicotine metabolism. FEBS Lett. 1999;460:321–327. doi: 10.1016/s0014-5793(99)01364-2. [DOI] [PubMed] [Google Scholar]
- 26.Mwenifumbo JC, Myers MG, Wall TL, Lin SK, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6*7, CYP2A6*8 and CYP2A6*10 as assessed with a novel haplotyping method. Pharmacogenet Genomics. 2005;15:189–192. doi: 10.1097/01213011-200503000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Fukami T, Nakajima M, Sakai H, McLeod HL, Yokoi T. CYP2A7 polymorphic alleles confound the genotyping of CYP2A6*4A allele. Pharmacogenomics J. 2006;6:401–412. doi: 10.1038/sj.tpj.6500390. [DOI] [PubMed] [Google Scholar]
- 28.Ho MK, Mwenifumbo JC, Zhao B, Gillam EM, Tyndale RF. A novel CYP2A6 allele, CYP2A6*23, impairs enzyme function in vitro and in vivo and decreases smoking in a population of Black-African descent. Pharmacogenet Genomics. 2008;18:67–75. doi: 10.1097/FPC.0b013e3282f3606e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu T, David SP, Tyndale RF, Wang H, Zhou Q, Ding P, et al. Associations of CYP2A6 genotype with smoking behaviors in southern China. Addiction. 2011;105:985–994. doi: 10.1111/j.1360-0443.2010.03353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd. Lippincott Williams & Wilkins; Philadelphia: 2008. p. 58. [Google Scholar]
- 31.Rose KM, Newman B, Mayer-Davis EJ, Selby JV. Genetic and behavioral determinants of waist-hip ratio and waist circumference in women twins. Obes Res. 1998;6:383–392. doi: 10.1002/j.1550-8528.1998.tb00369.x. [DOI] [PubMed] [Google Scholar]
- 32.Troisi RJ, Heinold JW, Vokonas PS, Weiss ST. Cigarette smoking, dietary intake, and physical activity: Effects on body fat distribution– the Normative Aging Study. Am J Clin Nutr. 1991;53:1104–1111. doi: 10.1093/ajcn/53.5.1104. [DOI] [PubMed] [Google Scholar]
- 33.Simon JA, Seeley DG, Lipschutz RC, Vittinghoff E, Browner WS. The relation of smoking to waist-to-hip ratio and diabetes mellitus among elderly women. Prev Med. 1997;26:639–644. doi: 10.1006/pmed.1997.0230. [DOI] [PubMed] [Google Scholar]
- 34.Akbartabartoori M, Lean ME, Hankey CR. Relationships between cigarette smoking, body size and body shape. Int J Obes. 2005;29:236–243. doi: 10.1038/sj.ijo.0802827. [DOI] [PubMed] [Google Scholar]
- 35.Shimokata H, Muller DC, Andres R. Studies in the distribution of body fat. III. Effects of cigarette smoking. JAMA. 1989;261:1169–1173. [PubMed] [Google Scholar]
- 36.Mizuno O, Okamoto K, Sawada M, Mimura M, Watanabe T, Morishita T. Obesity and smoking: Relationship with waist circumference and obesity-related disorders in men undergoing a health screening. J Atheroscler Thromb. 2005;12:199–204. doi: 10.5551/jat.12.199. [DOI] [PubMed] [Google Scholar]
- 37.Molenaar EA, Massaro JM, Jacques PF, Pou KM, Ellison RC, Hoffmann K, et al. Association of lifestyle factors with abdominal subcutaneous and visceral adiposity: The Framingham Heart Study. Diabetes Care. 2009;32:505–510. doi: 10.2337/dc08-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hideaki K, Yutaka M, Takuo YTN. Smoking as a Risk Factor for Visceral Fat Accumulation in Japanese Men. Tohoku J Exp Med. 2006;208:123–132. doi: 10.1620/tjem.208.123. [DOI] [PubMed] [Google Scholar]
- 39.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: A systematic review and meta-analysis. JAMA. 2007;298:2654–2664. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- 40.Koh-Banerjee P, Chu NF, Spiegelman D, Rosner B, Colditz G, Willett W, et al. Prospective study of the association of changes indietary intake, physical activity, alcohol consumption, and smoking with 9-y gain in waist circumference among 16 587 US men. Am J Clin Nutr. 2003;78:719–727. doi: 10.1093/ajcn/78.4.719. [DOI] [PubMed] [Google Scholar]
- 41.Rossini A, de Almeida Simao T, Albano RM, Pinto LF. CYP2A6 polymorphisms and risk for tobacco-related cancers. Pharmaco-genomics. 2008;9:1737–1752. doi: 10.2217/14622416.9.11.1737. [DOI] [PubMed] [Google Scholar]
- 42.Pianezza ML, Sellers EM, Tyndale RF. Nicotine metabolism defect reduces smoking. Nature. 1998;393:750. doi: 10.1038/31623. [DOI] [PubMed] [Google Scholar]
- 43.Tyndale RF, Sellers EM. Genetic variation in CYP2A6-mediated nicotine metabolism alters smoking behavior. Ther Drug Monit. 2002;24:163–171. doi: 10.1097/00007691-200202000-00026. [DOI] [PubMed] [Google Scholar]
- 44.Kamataki T, Fujieda M, Kiyotani K, Iwano S, Kunitoh H. Genetic polymorphism of CYP2A6 as one of the potential determinants of tobacco-related cancer risk. Biochem Biophys Res Commun. 2005;338:306–310. doi: 10.1016/j.bbrc.2005.08.268. [DOI] [PubMed] [Google Scholar]
- 45.Fujieda M, Yamazaki H, Saito T, Kiyotani K, Gyamfi MA, Sakurai M, et al. Evaluation of CYP2A6 genetic polymorphisms as determinants of smoking behavior and tobacco-related lung cancer risk in male Japanese smokers. Carcinogenesis. 2004;25:2451–2458. doi: 10.1093/carcin/bgh258. [DOI] [PubMed] [Google Scholar]
- 46.Derby KS, Cuthrell K, Caberto C, Carmella SG, Franke AA, Hecht SS, et al. Nicotine metabolism in three ethnic/racial groups with different risks of lung cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:3526–3535. doi: 10.1158/1055-9965.EPI-08-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tiwawech D, Srivatanakul P, Karalak A, Ishida T. Cytochrome P450 2A6 polymorphism in nasopharyngeal carcinoma. Cancer Lett. 2006;241:135–141. doi: 10.1016/j.canlet.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 48.Kadlubar S, Anderson JP, Sweeney C, Gross MD, Lang NP, Kadlubar FF, et al. Phenotypic CYP2A6 variation and the risk of pancreatic cancer. JOP. 2009;10:263–270. [PMC free article] [PubMed] [Google Scholar]
- 49.Song DK, Xing DL, Zhang LR, Li ZX, Liu J, Qiao BP. Association of NAT2, GSTM1, GSTT1, CYP2A6, and CYP2A13 gene polymorphisms with susceptibility and clinicopathologic characteristics of bladder cancer in Central China. Cancer Detect Prev. 2009;32:416–423. doi: 10.1016/j.cdp.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Ruwali M, Pant MC, Shah PP, Mishra BN, Parmar D. Polymorphism in cytochrome P450 2A6 and glutathione S-transferase P1 modifies head and neck cancer risk and treatment outcome. Mutat Res. 2009;669:36–41. doi: 10.1016/j.mrfmmm.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 51.Nakajima M, Kuroiwa Y, Yokoi T. Interindividual differences in nicotine metabolism and genetic polymorphisms of human CYP2A6. Drug Metab Rev. 2002;34:865–877. doi: 10.1081/dmr-120015696. [DOI] [PubMed] [Google Scholar]



