Abstract
An antigen immunologically related to a group-specific antigen (gp52, a 52,000-dalton glycoprotein) of the mouse mammary tumor virus has been identified in paraffin sections of human breast cancers by means of the indirect immunoperoxidase technique. The specificity of the reaction with antibody against mouse mammary tumor virus was examined by absorption of the IgG with the following: (a) purified gp52; (b) a number of virus preparations (mouse mammary tumor virus, Rauscher leukemia virus, simian sarcoma virus, baboon endogenous virus, and Mason—Pfizer monkey virus); (c) normal plasma, leukocytes, breast tissue, milk, actin, collagen, and hyaluronic acid, all of human origin; (d) sheep erythrocytes and mucin. Only mouse mammary tumor virus (from C3H or Paris RIII strains and grown in either murine or feline cells) and purified gp52 eliminated the immunohistochemical reaction in the human breast tumors.
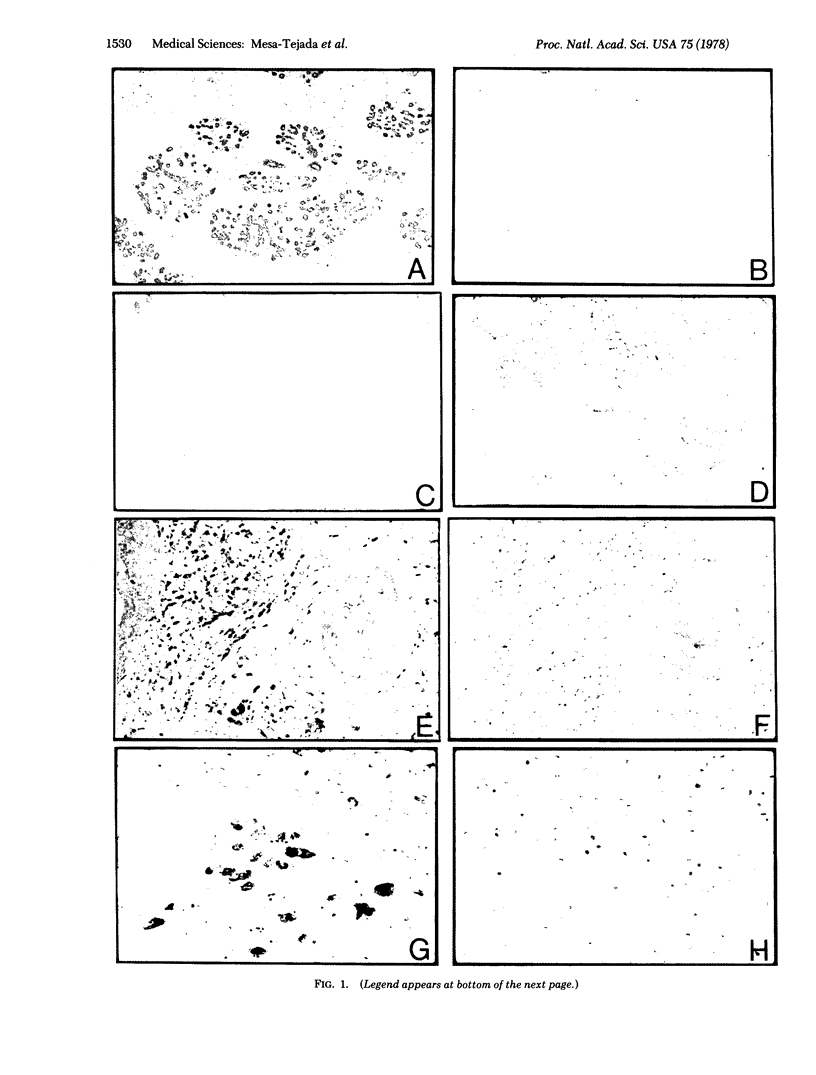
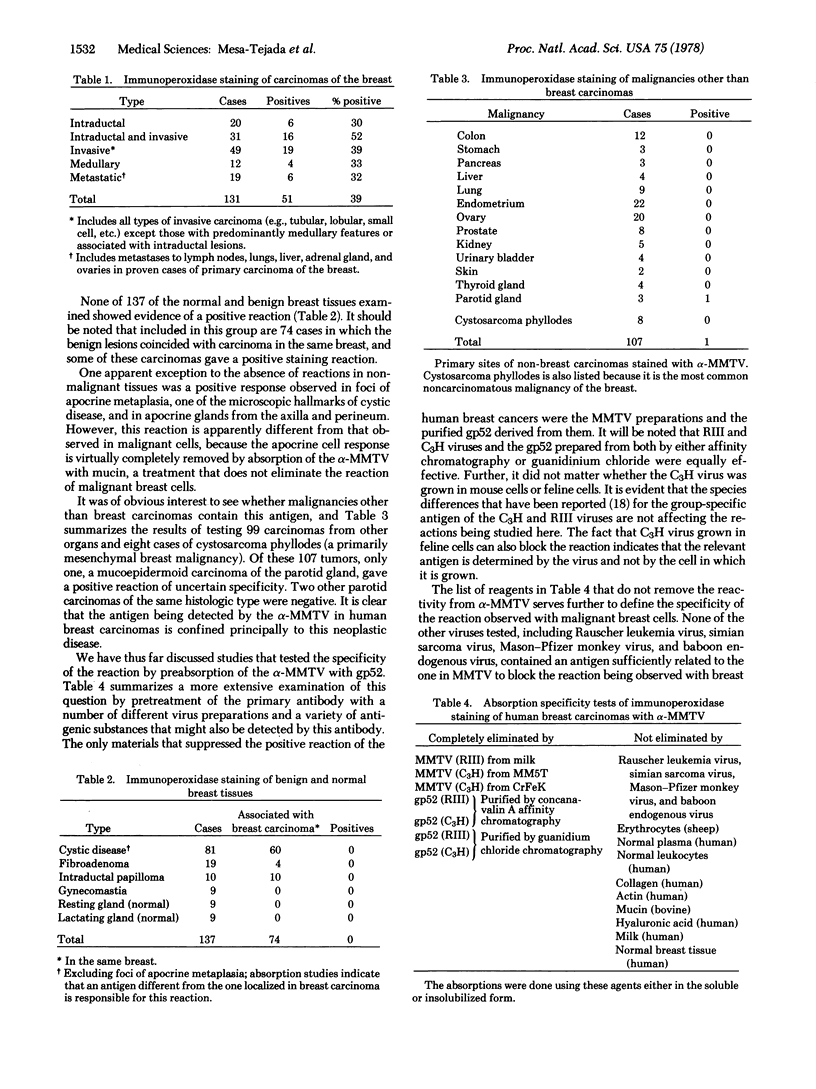
Positive reactions were seen in 51 of 131 (39%) breast carcinomas of various histologic types, a minimal estimate in view of the limited number of sections from each tumor that could be examined. Negative reactions were obtained in all 119 benign breast lesions (cystic disease, fibroadenoma, papilloma, gynecomastia) and in all 18 normal breast tissues. With one exception, 99 carcinomas from 13 organs other than breast and 8 cystosarcomas were all negative.
Keywords: immunoperoxidase staining, paraffin sections, cancer antigen
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Axel R., Gulati S. C., Spiegelman S. Particles containing RNA-instructed DNA polymerase and virus-related RNA in human breast cancers. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3133–3137. doi: 10.1073/pnas.69.11.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axel R., Schlom J., Spiegelman S. Presence in human breast cancer of RNA homologous to mouse mammary tumour virus RNA. Nature. 1972 Jan 7;235(5332):32–36. doi: 10.1038/235032a0. [DOI] [PubMed] [Google Scholar]
- Black M. M., Zachrau R. E., Shore B., Leis H. P., Jr Biological considerations of tumor-specific and virus-associated antigens of human breast cancers. Cancer Res. 1976 Feb;36(2 Pt 2):769–774. [PubMed] [Google Scholar]
- Bowen J. M., Dmochowski L., Miller M. F., Priori E. S., Seman G., Dodson M. L., Maruyama K. Implications of humoral antibody in mice and humans to breast tumor and mouse mammary tumor virus-associated antigens. Cancer Res. 1976 Feb;36(2 Pt 2):759–764. [PubMed] [Google Scholar]
- Charney J., Moore D. H. Neutralization of murine mammary tumour virus by sera of women with breast cancer. Nature. 1971 Feb 26;229(5287):627–628. doi: 10.1038/229627a0. [DOI] [PubMed] [Google Scholar]
- Colcher D., Spiegelman S., Schlom J. Sequence homology between the RNA of Mason-Pfizer monkey virus and the RNA of human malignant breast tumors. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4975–4979. doi: 10.1073/pnas.71.12.4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchimont P., Zangerle P. F., Hendrick J. C., Reuter A., Colin C. Simultaneous assays of cancer associated antigens in benign and malignant breast diseases. Cancer. 1977 Jun;39(6 Suppl):2806–2812. doi: 10.1002/1097-0142(197706)39:6<2806::aid-cncr2820390668>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Green R. W., Bolognesi D. P. Isolation of proteins by gel filtration in 6M guanidinium chloride: application to RNA tumor viruses. Anal Biochem. 1974 Jan;57(1):108–117. doi: 10.1016/0003-2697(74)90057-8. [DOI] [PubMed] [Google Scholar]
- Holder W. D., Jr, Peer G. W., Bolognesi D. P., Wells S. A., Jr Antibody reacting with mouse mammary tumor virus in serum of breast carcinoma patients. Surg Forum. 1976;27(62):102–104. [PubMed] [Google Scholar]
- Hosaka Y., Hosokawa Y. Purification of Sendai virions with glutaraldehyde-treated red blood cells. Intervirology. 1977;8(1):1–17. doi: 10.1159/000148872. [DOI] [PubMed] [Google Scholar]
- Keydar I., Mesa-Tejada R., Ramanarayanan M., Ohno T., Fenoglio C., Hu R., Spiegelman S. Detection of viral proteins in mouse mammary tumors by immunoperoxidase staining of paraffin sections. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1524–1528. doi: 10.1073/pnas.75.3.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesa-Tejada R., Pascal R. R., Fenoglio C. M. Immunoperoxidase: a sensitive immunohistochemical technique as a "special stain" in the diagnostic pathology laboratory. Hum Pathol. 1977 May;8(3):313–320. doi: 10.1016/s0046-8177(77)80028-2. [DOI] [PubMed] [Google Scholar]
- Michalides R., Spiegelman S., Schlom J. Biochemical characterization of putative subviral particulates from human malignant breast tumors. Cancer Res. 1975 Apr;35(4):1003–1008. [PubMed] [Google Scholar]
- Müller M., Kemmer C., Zotter S., Grobbman H., Micheel B. Kreuzreaktion zwischen Brustkrebs und Mastopathie des Menschen sowie murinen Mammakarzinomen: Lokalisation des Antigens im Bereich de A-Partikel des Mammatumorvirus. Arch Geschwulstforsch. 1973;41(2):100–106. [PubMed] [Google Scholar]
- Müller M., Zotter S., Grossmann H., Kemmer C. Immunologische Kreuzreaktion zwischen Brustkrebs und Mastopathie des Menschen sowie virusproduzierenden Mammakarzinomen der Maus. Arch Geschwulstforsch. 1972;40(4):285–299. [PubMed] [Google Scholar]
- Ohno T., Spiegelman S. Antigenic relatedness of the DNA polymerase of human breast cancer particles to the enzyme of the Mason-Pfizer monkey virus. Proc Natl Acad Sci U S A. 1977 May;74(5):2144–2148. doi: 10.1073/pnas.74.5.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascal R. R., Mesa-Tejada R., Bennett S. J., Garces A., Fenoglio C. M. Carcinoembryonic antigen: immunohistologic identification in invasive and intraepithelial carcinomas of the lung. Arch Pathol Lab Med. 1977 Nov;101(11):568–571. [PubMed] [Google Scholar]
- Ritzi E., Baldi A., Spiegelman S. The purification of a gs antigen of the murine mammary tumor virus and its quantitation by radioimmunoassay. Virology. 1976 Nov;75(1):188–197. doi: 10.1016/0042-6822(76)90017-9. [DOI] [PubMed] [Google Scholar]
- Ritzi E., Martin D. S., Stolfi R. L., Spiegelman S. Plasma levels of a viral protein as a diagnostic signal for the presence of mammary tumor: the effect of tumor removal. J Exp Med. 1977 Apr 1;145(4):999–1013. doi: 10.1084/jem.145.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman S., Axel R., Schlom J. Virus-related RNA in human and mouse mammary tumors. J Natl Cancer Inst. 1972 Apr;48(4):1205–1211. [PubMed] [Google Scholar]
- Teramoto Y. A., Kufe D., Schlom J. Type-specific antigenic determinants on the major external glycoprotein of high- and low-oncogenic murine mammary tumor viruses. J Virol. 1977 Nov;24(2):525–533. doi: 10.1128/jvi.24.2.525-533.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya A. B., Black M. M., Dion A. S., Moore D. H. Homology between human breast tumour RNA and mouse mammary tumour virus genome. Nature. 1974 Jun 7;249(457):565–567. doi: 10.1038/249565a0. [DOI] [PubMed] [Google Scholar]