Abstract
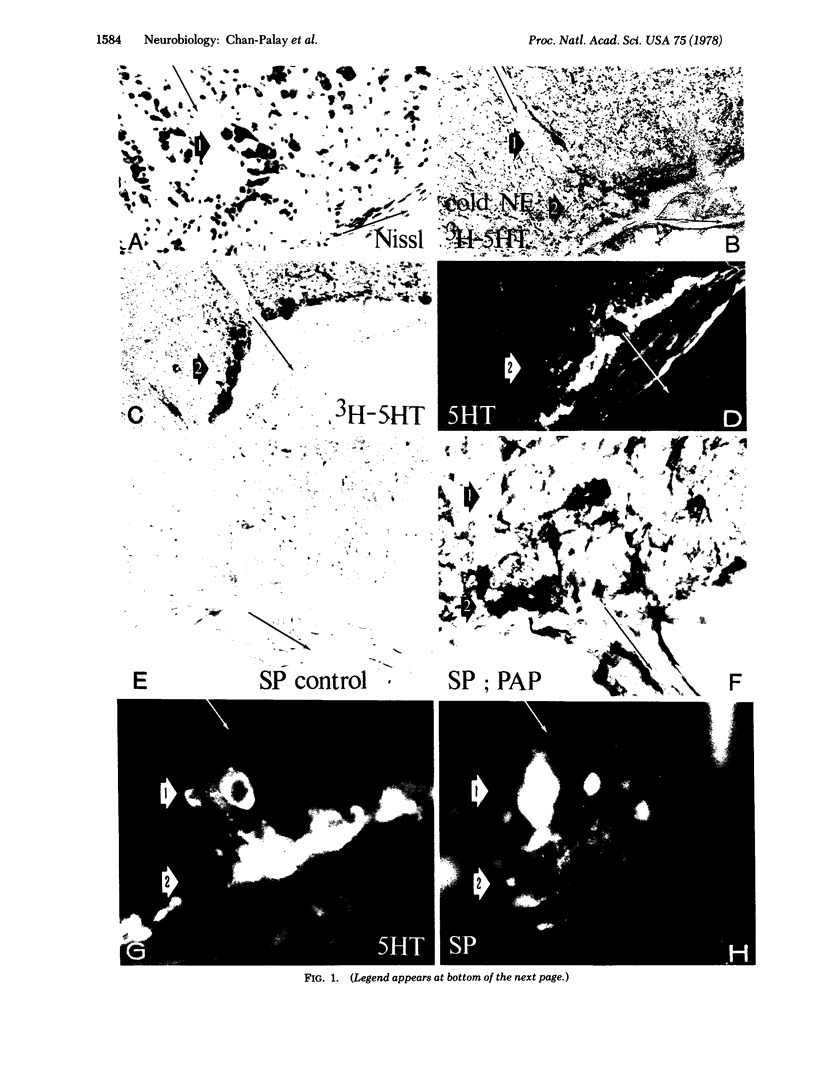
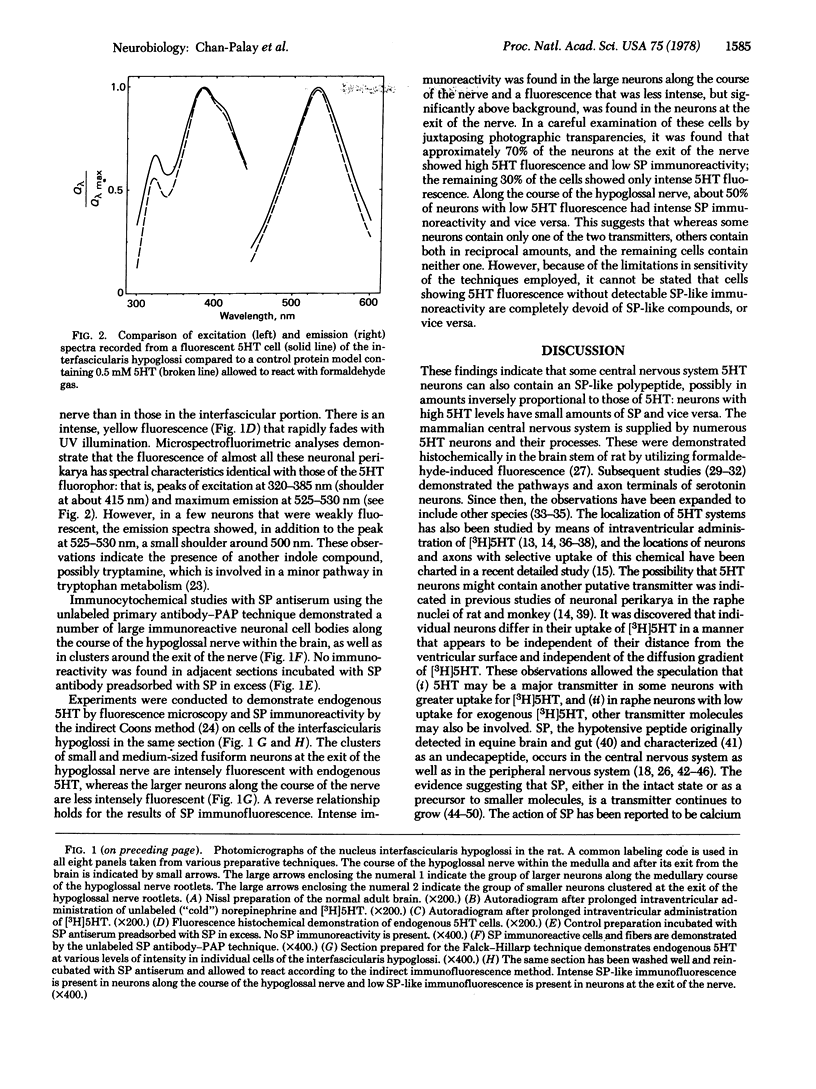
5-Hydroxytryptamine (serotonin)-containing neurons in the rat's medullary raphe and interfascicularis hypoglossi cell groups were identified by means of autoradiography following prolonged intraventricular administration of 5-hydroxy[3H]tryptamine, fluorescence histochemistry for the demonstration of endogenous 5-hydroxytryptamine, and microspectrofluorimetric analysis of excitation and emission spectra. Immunocytochemical methods (the unlabeled primary antibody—peroxidase antiperoxidase and indirect immunofluorescence methods) were applied with antisera to substance P in order to localize immunoreactivity in these medullary neurons. It was demonstrated that the raphe nuclei and the interfascicularis hypoglossi nucleus are heterogeneous cell groups that contain: (i) Neurons that display both an uptake—storage capacity for 5-hydroxy[3H]tryptamine and a formaldehyde-induced fluorescence with spectral characteristics identical to those of the 5-hydroxytryptamine fluorophor. These cells exhibit high to low fluorescence intensities without detectable substance P-like immunoreactivity. (ii) Neurons with various 5-hydroxytryptamine fluorescence intensities and intense to low degrees of substance P-like immunoreactivity. (iii) Neurons with various degrees of substance P-like immunoreactivity without detectable 5-hydroxytryptamine fluorescence or 5-hydroxy[3H]tryptamine uptake and storage capacity. These results indicate that some neurons contain high or low levels of only 5-hydroxytryptamine or substance P, whereas other neurons contain both 5-hydroxytryptamine and substance P in various proportions. The present findings demonstrate the presence of two putative transmitters, a biogenic amine and a polypeptide, within the same neuron in the mammalian central nervous system.
Keywords: autoradiography, fluorescence histochemistry, microspectrofluorimetry, immunofluorescence, unlabeled antibody—peroxidase, antiperoxidase
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agranoff B. W. Neurotransmitters and synaptic transmission. Fed Proc. 1975 Sep;34(10):1911–1914. [PubMed] [Google Scholar]
- Björklund A., Falck B., Stenevi U. Classification of monoamine neurones in the rat mesencephalon: distribution of a new monoamine neurone system. Brain Res. 1971 Sep 24;32(2):269–285. doi: 10.1016/0006-8993(71)90324-6. [DOI] [PubMed] [Google Scholar]
- Bloom F. E., Hoffer B. J., Siggins G. R., Barker J. L., Nicoll R. A. Effects of serotonin on central neurons: microiontophoretic administration. Fed Proc. 1972 Jan-Feb;31(1):97–106. [PubMed] [Google Scholar]
- Borys H. K., Weinreich D., McCaman R. E. Determination of glutamate and glutamine in individual neurons of Aplysia californica. J Neurochem. 1973 Nov;21(5):1349–1351. doi: 10.1111/j.1471-4159.1973.tb07590.x. [DOI] [PubMed] [Google Scholar]
- Brownstein M. J., Saavedra J. M., Axelrod J., Zeman G. H., Carpenter D. O. Coexistence of several putative neurotransmitters in single identified neurons of Aplysia. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4662–4665. doi: 10.1073/pnas.71.12.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Do some nerve cells release more than one transmitter? Neuroscience. 1976 Aug;1(4):239–248. doi: 10.1016/0306-4522(76)90054-3. [DOI] [PubMed] [Google Scholar]
- COONS A. H. Fluorescent antibody methods. Gen Cytochem Methods. 1958;1:399–422. [PubMed] [Google Scholar]
- Chan-Palay V. Fine structure of labelled axons in the cerebellar cortex and nuclei of rodents and primates after intraventricular infusions with tritiated serotonin. Anat Embryol (Berl) 1975 Dec 31;148(3):235–265. doi: 10.1007/BF00319846. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V. Indoleamine neurons and their processes in the normal rat brain and in chronic diet-induced thiamine deficiency demonstrated by uptake of 3H-serotonin. J Comp Neurol. 1977 Dec 15;176(4):467–493. doi: 10.1002/cne.901760402. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V., Palay S. L. Immunocytochemical identification of substance P cells and their processes in rat sensory ganglia and their terminals in the spinal cord: light microscopic studies. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3597–3601. doi: 10.1073/pnas.74.8.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Palay V. Serotonin axons in the supra- and subependymal plexuses and in the leptomeninges; their roles in local alterations of cerebrospinal fluid and vasomotor activity. Brain Res. 1976 Jan 30;102(1):103–130. doi: 10.1016/0006-8993(76)90578-3. [DOI] [PubMed] [Google Scholar]
- Chang M. M., Leeman S. E., Niall H. D. Amino-acid sequence of substance P. Nat New Biol. 1971 Jul 21;232(29):86–87. doi: 10.1038/newbio232086a0. [DOI] [PubMed] [Google Scholar]
- Cottrell G. A. Proceedings: Does the giant cerebral neurone of Helix release two transmitters: ACh and serotonin? J Physiol. 1976 Jul;259(1):44P–45P. [PubMed] [Google Scholar]
- Davies J., Dray A. Substance P in the substantia nigra. Brain Res. 1976 May 14;107(3):623–627. doi: 10.1016/0006-8993(76)90150-5. [DOI] [PubMed] [Google Scholar]
- Einarsson P., Hallman H., Jonsson G. Quantitative microfluorimetry of formaldehyde induced fluorescence of dopamine in the caudate nucleus. Med Biol. 1975 Feb;53(1):15–24. [PubMed] [Google Scholar]
- FUXE K. EVIDENCE FOR THE EXISTENCE OF MONOAMINE NEURONS IN THE CENTRAL NERVOUS SYSTEM. IV. DISTRIBUTION OF MONOAMINE NERVE TERMINALS IN THE CENTRAL NERVOUS SYSTEM. Acta Physiol Scand Suppl. 1965:SUPPL 247–247:37+. [PubMed] [Google Scholar]
- Felten D. L., Laties A. M., Carpenter M. B. Monoamine-containing cell bodies in the squirrel monkey brain. Am J Anat. 1974 Feb;139(2):153–165. doi: 10.1002/aja.1001390202. [DOI] [PubMed] [Google Scholar]
- Fuxe K., Hökfelt T., Ungerstedt U. Localization of indolealkylamines in CNS. Adv Pharmacol. 1968;6(Pt A):235–251. doi: 10.1016/s1054-3589(08)61177-1. [DOI] [PubMed] [Google Scholar]
- Fuxe K., Jonsson G. A modification of the histochemical fluorescence method for the improved localization of 5-hydroxytryptamine. Histochemie. 1967;11(2):161–166. doi: 10.1007/BF00571721. [DOI] [PubMed] [Google Scholar]
- Fuxe K., Jonsson G. The histochemical fluorescence method for the demonstration of catecholamines. Theory, practice and application. J Histochem Cytochem. 1973 Apr;21(4):293–311. doi: 10.1177/21.4.293. [DOI] [PubMed] [Google Scholar]
- Fuxe K., Ungerstedt U. Histochemical studies on the distribution of catecholamines and 5-hydroxytryptamine after intraventricular injections. Histochemie. 1968;13(1):16–28. doi: 10.1007/BF00303872. [DOI] [PubMed] [Google Scholar]
- Henry J. L., Krnjevíc K., Morris M. E. Substance P and spinal neurones. Can J Physiol Pharmacol. 1975 Jun;53(3):423–432. doi: 10.1139/y75-061. [DOI] [PubMed] [Google Scholar]
- Hubbard J. E., Di Carlo V. Fluorescence histochemistry of monoamine-containing cell bodies in the brain stem of the squirrel monkey (Saimiri sciureus). 3. Serotonin-containing groups. J Comp Neurol. 1974 Feb 15;153(4):385–398. doi: 10.1002/cne.901530404. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Elde R., Johansson O., Luft R., Nilsson G., Arimura A. Immunohistochemical evidence for separate populations of somatostatin-containing and substance P-containing primary afferent neurons in the rat. Neuroscience. 1976;1(2):131–136. doi: 10.1016/0306-4522(76)90008-7. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Elfvin L. G., Elde R., Schultzberg M., Goldstein M., Luft R. Occurrence of somatostatin-like immunoreactivity in some peripheral sympathetic noradrenergic neurons. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3587–3591. doi: 10.1073/pnas.74.8.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T., Kellerth J. O., Nilsson G., Pernow B. Substance p: localization in the central nervous system and in some primary sensory neurons. Science. 1975 Nov 28;190(4217):889–890. doi: 10.1126/science.242075. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Jessell T., Kanazawa I. Release and metabolism of substance P in rat hypothalamus. Nature. 1976 Nov 4;264(5581):81–83. doi: 10.1038/264081a0. [DOI] [PubMed] [Google Scholar]
- Jonsson G., Einarsson P., Fuxe K., Hallman H. Microspectrofluorimetric analysis of the formaldehyde induced fluorescence in midbrain raphe neurons. Med Biol. 1975 Feb;53(1):25–39. [PubMed] [Google Scholar]
- Kerkut G. A., Sedden C. B., Walker R. J. Uptake of DOPA and 5-hydroxytryptophan by monoamine-forming neurones in the brain of Helix aspersa. Comp Biochem Physiol. 1967 Oct;23(1):159–162. doi: 10.1016/0010-406x(67)90483-5. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Morris M. E. An excitatory action of substance P on cuneate neurones. Can J Physiol Pharmacol. 1974 Jun;52(3):736–744. doi: 10.1139/y74-094. [DOI] [PubMed] [Google Scholar]
- Otsuka M., Konishi S. Release of substance P-like immunoreactivity from isolated spinal cord of newborn rat. Nature. 1976 Nov 4;264(5581):83–84. doi: 10.1038/264083a0. [DOI] [PubMed] [Google Scholar]
- Otsuka M., Konishi S. Substance P and excitatory transmitter of primary sensory neurons. Cold Spring Harb Symp Quant Biol. 1976;40:135–143. doi: 10.1101/sqb.1976.040.01.015. [DOI] [PubMed] [Google Scholar]
- Owman C., Håkanson R., Sundler F. Occurrence and function of amines in endocrine cells producing polypeptide hormones. Fed Proc. 1973 Jul;32(7):1785–1791. [PubMed] [Google Scholar]
- Pearse A. G. The cytochemistry and ultrastructure of polypeptide hormone-producing cells of the APUD series and the embryologic, physiologic and pathologic implications of the concept. J Histochem Cytochem. 1969 May;17(5):303–313. doi: 10.1177/17.5.303. [DOI] [PubMed] [Google Scholar]
- Pickel V. M., Joh T. H., Reis D. J. Monoamine-synthesizing enzymes in central dopaminergic, noradrenergic and serotonergic neurons. Immunocytochemical localization by light and electron microscopy. J Histochem Cytochem. 1976 Jul;24(7):792–306. doi: 10.1177/24.7.8567. [DOI] [PubMed] [Google Scholar]
- Steinacker A., Highstein S. M. Pre- and postsynaptic action of substance P at the Mauther fiber-giant fiber synapse in the hatchetfish. Brain Res. 1976 Sep 10;114(1):128–133. doi: 10.1016/0006-8993(76)91013-1. [DOI] [PubMed] [Google Scholar]
- V Euler U. S., Gaddum J. H. An unidentified depressor substance in certain tissue extracts. J Physiol. 1931 Jun 6;72(1):74–87. doi: 10.1113/jphysiol.1931.sp002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J. H., Williams L. D. Monoamine-containing neurons in planaria. J Comp Neurol. 1970 Jan;138(1):103–115. doi: 10.1002/cne.901380108. [DOI] [PubMed] [Google Scholar]