Figure 1. Drp1 promotes Bax oligomerization in the presence of tBid.
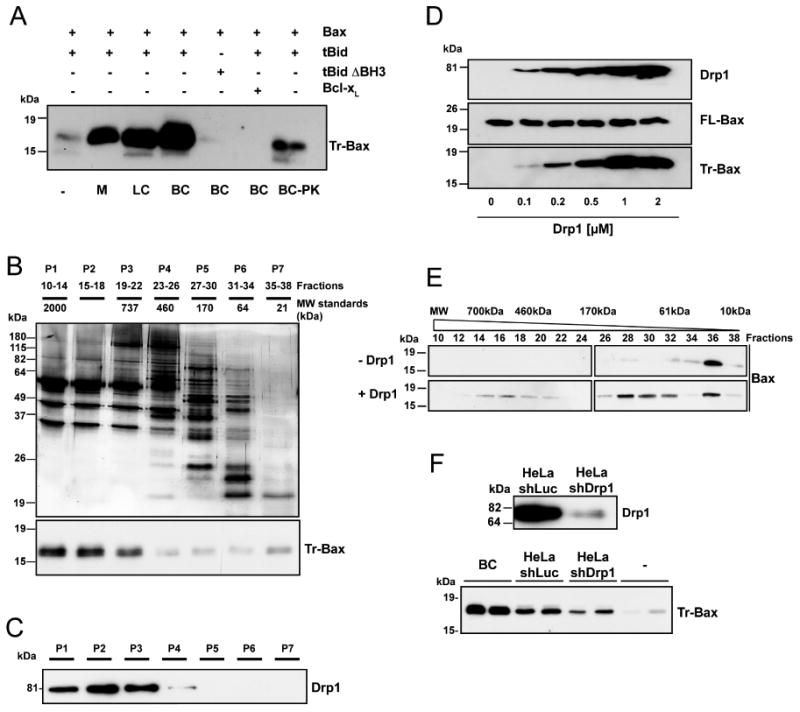
(A) PC/PE/CL (54/20/26 mol%) liposomes were incubated with the indicated proteins at 30°C for 30 min before ultracentrifugation, resuspension in KCl buffer and incubation with trypsin for 2 h at 30°C. Trypsin-resistant Bax (Tr-Bax) was analyzed by immunoblotting. M: salt-extracted liver mitochondrial proteins (400 μg); LC: rat liver cytosolic extract (200 μg); BC: rat brain cytosolic extract (200 μg); BC-PK: BC (200 μg) treated with proteinase K. Bax (50 nM); tBid (10 nM); tBid ΔBH3 (10 nM); Bcl-xL (1 μM). The blot is representative of three independent experiments.
(B) Purification steps of Bax-activating proteins: silver stained SDS-polyacrylamide gel electrophoresis of BC proteins fractionated by size exclusion chromatography (top panel); Bax-activating proteins (assessed as in A) are found in P1-P3 fractions (lower panel). Blots are representative of at least three independent experiments. P1 displayed the highest specific BAF activity and was found to contain GAPDH, Aldolase, Tubulin, Microtubule Associated Protein 1, Dynein, Spectrin, Actin, Gelsolin, Alpha Actinin, Cofilin and Drp1. Except Drp1, all proteins were found to be inactive in the Bax oligomerization assay.
(C) Immunoblot showing that Drp1 is present in P1-P3 fractions.
(D) Dose-response analysis of in vitro tBid-induced Bax oligomerization with increasing concentrations of recombinant Drp1. PC/PE/CL liposomes were incubated with 10 nM tBid and 50 nM Bax and increasing amounts of recombinant Drp1 before trypsin digestion and Bax analysis. Upper immunoblots show levels of Drp1 and Bax before trypsin digestion. The blot is representative of three independent experiments.
(E) Analysis of tBid-induced Bax oligomerization in isolated liposomes in the absence or presence of 1 μM Drp1 by size exclusion chromatography. PC/PE/CL liposomes were incubated with 10 nM tBid, 50 nM Bax and 1 μM Drp1. Liposomes were then lysed in 2% CHAPS, 200 nM NaCl, and proteins fractionated by size exclusion chromatography. The elution profile of Bax was analyzed by immunoblotting.
(F) Upper panel: Western blot analysis of Drp1 in control (shLuc) and Drp1-depleted (shDrp1) HeLa cells. Lower panel: analysis of Bax activating capacity of control and Drp1-depleted HeLa cytosolic extracts (200 μg each). Brain cytosol (BC) was also tested at 200 μg. In each experiment, the same cytosolic extract from HeLa cells was tested in duplicate. The experiment was repeated twice, using the same cell lines but with a different cytosolic extract preparation.
