Figure 7. Expression of Drp1 R247A/E delays cell death.
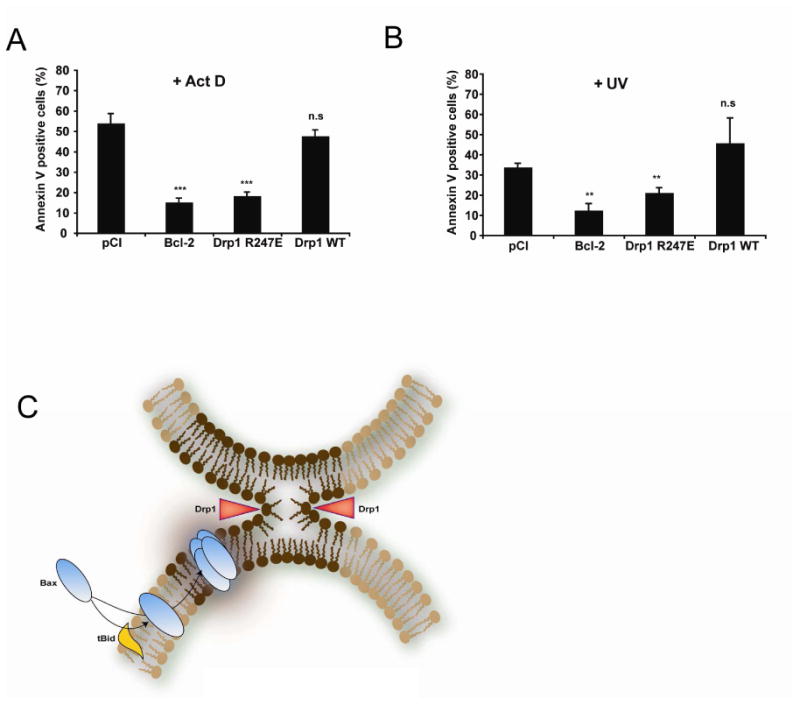
(A, B) HeLa cells were transfected with an empty vector (pCI) or with plasmids encoding Bcl-2, Drp1 WT or Drp1 R247E. 72 h later, cell cultures were treated with 3 μM Act D (A) or UV- irradiated (60 mJ/cm2) (B) and apoptosis quantified 6 h later by Annexin V staining and FACS analysis. Values are the average of six independent experiments ± S.E. **: p < 0.01; ***: p < 0.001; (n.s.), not significant.
(C) Model explaining the role of mitochondrial membrane hemifission or hemifusion intermediates in Bax oligomerization. During apoptosis, Bax is recruited to the outer mitochondrial membrane by tBid (or other BH3 only proteins) where it inserts. At the same time, Drp1 constricts the organelle as indicated by red arrowheads, triggering the formation of a hemifission intermediate. This membrane remodeling (dark brown part of the membrane) promotes Bax oligomerization. We speculate that contact sites between the inner and outer membranes, that are enriched in CL, could also be privileged sites for the formation of hemifusion intermediates, independently of Drp1. Contact sites could therefore represent additional sites in which Bax oligomerization would occur.
