Fig. 4.
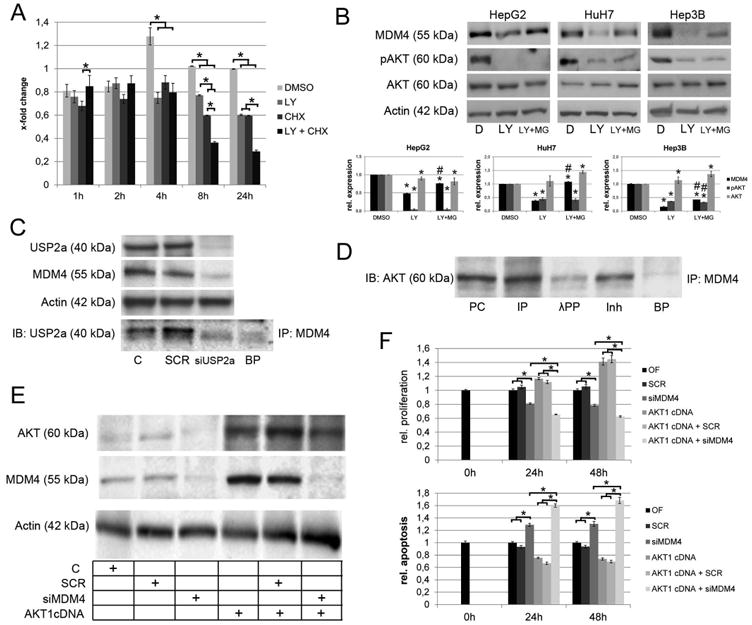
Mechanisms involved in the post-transcriptional regulation of MDM4. (A) Densitometric analysis of the immunoblots following LY294002 and/or CHX treatment. AKT signaling promotes stabilization of the MDM4 protein as indicated by significantly reduced MDM4 protein levels following LY294002 treatment of HCC cells with CHX-induced termination of protein biosynthesis (8h: 0.60 ± 0.001 compared to 0.36 ± 0.001 in CHXtreated controls, P<.05; 24 h: 0.60 ± 0.01 vs. 0.29 ± 0.01, respectively, P<.05). (B) Inhibition of proteasomal degradation rescues the MDM4 protein levels after LY294002 treatment of HCC cells. Lower panel: densitometric analysis of Western blots. *DMSO vs. LY294002 or LY294002 + MG132, P < .05; #LY294002 vs. LY294002 + MG132, P < .05 (C) USP2a physically interacts with MDM4 and siRNA-mediated silencing of USP2a reduces the MDM4 protein levels indicating that USP2a-mediated deubiquitination is involved in the stabilization of the MDM4 protein. (D) Co-immunoprecipitation demonstrating a physical interaction between AKT and MDM4 in HCC cells. Preincubation with lambda protein phosphatase reduces the AKT-MDM4 interaction. (E) Western immunobloting following transfection of in HLE cells using an AKT1 cDNA with/without simultaneous siRNA inhibition of MDM4. (F) Transfection of AKT1 increase the proliferation (upper panel) and decreases the apoptotic rate (lower panel), which can be rescued by siRNAmediated knockdown of MDM4. Abbreviations: BP, blocking peptide; C, untreated control; CHX, cycloheximide; D, DMSO; IB, immunoblot; Inh, inhibitor; incubation with lambda protein phosphatase and phosphatase inhibitor; IP, immunoprecipitation; λPP, lambda protein phosphatase; LY, LY294002; MG, MG132; SCR, scrambled siRNA; PC, positive control; siUSP2a, siRNA targeting USP2a.
