Abstract
Contemporary clinical practice for the care of the prematurely born babies has markedly improved their rates of survival so that most of these babies are expected to grow up to live a healthy functional life. Since the clinical follow up is of short duration (years), only limited data are available to relate non-communicable diseases in adult life to events and interventions in the neonatal period. The major events that could have a programming effect include (1) Intrauterine growth restriction (2) Interruption of pregnancy with change in redox and reactive oxygen species injury (3) Nutritional and pharmacological protocols for Clinical care (4) Nutritional care in the first two years resulting in accelerated weight gain. The available data are discussed in the context of perturbations in one carbon (methyl transfer) metabolism and its possible programming effects. Although direct evidence for genomic methylation is not available, clinical and experimental data on impact of redox and ROS, of low protein intake, excess methionine load and vitamin A, on methyl transfers are reviewed. The consequences of antenatal and postnatal administration of glucocorticoids are presented. Analysis of the correlates of insulin sensitivity at older age, suggests that premature birth is the major contributor, and is compounded by gain in weight during infancy. We speculate that premature interruption of pregnancy and neonatal interventions by effecting one carbon metabolism may cause programming effects on the immature baby. These can be additive to the effects of intrauterine environment (growth restriction) and are compounded by accelerated growth in early infancy.
Keywords: prematurity, programming, methionine, insulin resistance, parenteral nutrition, vitamin A
Contemporary neonatal intensive care methods have resulted in dramatic improvements in the survival of the low birth weight (LBW) infants (<1500 grams) (1,2). Since 2000, however, the overall survival rate has remained stable and no additional improvement in these rates has occurred. (Table 1) During the 1990s, the clinical approach for the care of the LBW infants became more aggressive with increased use of antenatal and postnatal steroids, cesarean section delivery, surfactant therapy, assisted ventilation and the use of a number of pharmacological interventions some tested and others untested in this population. These have been associated with an increase in morbidities including bronchopulmonary dysplasia, sepsis, and high rates of neurodevelopmental impairment (3,4). Since these developments in clinical care and therefore improvement in survival rates have been recent in terms of chronology, these babies are only in the twenties or thirties; there are insufficient data to relate the development of non-communicable disease of adults to the nutritional, environmental and other influences early in life. As the oldest preterm survivors enter young adulthood, the vast majority of them live free from neurosensory impairment but may suffer from a variety of other medical problems such as asthma, hypertension, obesity, type 2 diabetes, hyperlipidemia and cancer (5,6,7). Furthermore prematurity has been implicated in the origin of adult depression and behavioral problems (3,4). Whether these non-communicable disorders are the consequence of programming remains to be determined. Data from studies in experimental animals showing a relationship between changes in intrauterine and neonatal nutritional and other environmental perturbations and long term consequences in the offspring suggest that such may be the case also in the prematurely born human newborn.
Table 1. Rainbow Babies and Children’s Hospital Neonatal Intensive Care Admission and Survival Rates by Gestational Age for Children with Birth Weight <1000 grams.
| Weeks GA | 1982-1989 | 1990-1999 | 2000-2007 | |||
|---|---|---|---|---|---|---|
| Admits, n (%) | % Survival | Admits, n (%) | % Survival | Admits, n (%) | % Survival | |
| 21-24 wks | 61 (15%) | 39% | 165 (25%) | 53% | 125 (25%) | 59% |
| 25-27 wks | 245 (60%) | 57% | 393 (59%) | 83% | 290 (59%) | 88% |
| 28+ wks | 105 (25%) | 74% | 104 (15%) | 92% | 80 (15%) | 89% |
The evaluation of programming effects in prematurely born babies is difficult because of lack of large prospective data specifically in relation to the development of non-communicable diseases. However identification of potential contributors for whom animal data exists, for example influence of methyl group load, or the effects of pharmacological interventions like vitamin A, glucocorticoids etc., can help identify programming related changes. Finally the available outcome data can be correlated retrospectively to the potential events early in life that could have programming effect. However, the changes in demography of the study population in combination with changes in the algorithms of clinical care have made such evaluation difficult. In the present review we will discuss the available data in humans with particular emphasis on the contribution of interventions in the neonatal intensive care unit (NICU). Where applicable, reference is made to the contributing data from studies in animal models. However it should be underscored that premature birth, neonatal intensive care and survival of extremely immature babies are phenomena that are historically recent and unique to humans. Until recently there were no animal models of prematurity and the data of animal models of premature birth were limited and exclusively of short duration.
The major events that could potentially result in programming effects in a prematurely born infant can be listed as follows:
Intrauterine environment
Premature interruption of pregnancy
Neonatal interventions
Nutritional care during infancy
Contributors to Programming(1): Intrauterine environment
The critical role of intrauterine environment on fetal growth and programming has been documented in a large number of studies in humans and in animal models (8-10). Data from studies in animal models show that various nutrient and environmental manipulations during pregnancy result in fetal growth restriction, lower birth weight and programming of the offspring (8,10). Studies in humans have confirmed the original observation of Barker and colleagues that intrauterine growth restriction as evidenced by lower weight at birth is associated with the higher incidence of non-communicable disease in the offspring in adult life (9). In this context, it is interesting to note that a significantly high proportion of low birth weight babies have been reported to be growth retarded in utero and born small for gestational age in various studies (11). However, the long term programming consequence of the intrauterine growth restriction early in gestation cannot be easily separated from the confounding influence of other contributors to programming, particularly in studies in humans.
Contributors to Programming(2): Interruption of Pregnancy
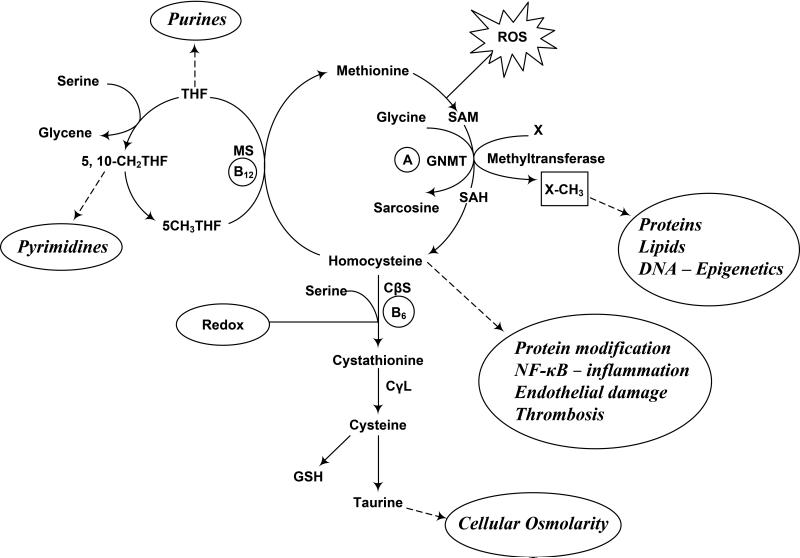
The impact of premature interruption of pregnancy on the immature baby remains undefined. The transition from fetus to neonate is accompanied by changes in respiration, circulation, glucose and energy homeostasis, thermoregulation and a host of other endocrine, metabolic and physical responses that allow for independent extra-uterine existence. Significantly, there is a marked change in the redox state and oxygenation from a relative hypoxic and markedly reduced fetal state to a highly oxygenated extra-uterine life (12,13). These changes are accompanied by generation of reactive oxygen species (ROS) and consequently changes in metabolism to compensate for the oxidant insult. ROS can cause oxidative modification of major cellular macromolecules i.e. lipids, proteins and DNA. A number of molecular targets of ROS have been identified (14) In the context of programming, one carbon metabolism or the methyl transfer plays a critical role in the methylation of proteins, lipids and the methylation of DNA in the transcriptional control of gene expression and metabolism (15). The key constituent parts of the one carbon metabolism are displayed in figure 1. Folate and methionine cycle form the key components of the one carbon metabolism or methyl transfers and are present ubiquitously in every cell in the body. Methyl groups from serine and glycine entering the folate cycle, are transferred to homocysteine to form methionine. Methionine, an essential amino acid, is the immediate source of the methyl groups required for the methylation of proteins, nucleic acids, biogenic amines and phospholipids etc. Methionine is converted to the bioactive compound, s-adenosylmethionine (SAM), the ubiquitous methyl donor, catalyzed by methionine adenosyl transferase. The transfer of methyl groups from SAM is catalyzed by several different methyl transferases and result in the formation of s-adensylhomocysteine and subsequently homocysteine. Homocysteine can either be methylated back to methionine or can enter the methionine catabolic pathway, the transsulfuration cascade, wherein it condenses with serine to form cystathionine. Cystathionine, through a series of metabolic steps is converted to cysteine and ultimately to taurine. As shown in the figure, the reactive oxygen species and change in redox can influence the key regulatory steps of methionine metabolism i.e. the synthesis of s-adenosylmethionine and the transulffuration cascade involved in the synthesis of cysteine and ultimately glutathione (16-18). Changes in redox and reactive oxygen species could potentially affect the generation of SAM and glutathione and consequently impact the methylation process. Although not studied, perturbations in the one carbon metabolism at the time of birth could have long lasting effect on gene expression and cause programming in the immature baby. It is important to underscore that the expression of genes regulating metabolism is an extremely orchestrated phenomenon, so that several of the metabolic processes appear at critical predetermined points during development. For example, hepatic cytosolic PEPCK and gluconeogenesis appears for the first time after birth in rodent and other mammalian species (19). Similarly cystathionine gamma lyase is not present in fetal liver and appears after birth (20). Premature interruption of pregnancy and premature birth will result in early expression of these pathways for independent existence. The long term consequence of these changes or programming is not known. This could be akin to modification of gene expression demonstrated in the newborn rats as a result of changes in the diet in the neonatal period (21).
Fig. 1.
The one carbon (methyl group) metabolism in vivo. The interrelationship between folate and methionine metabolism and its regulation are displayed. As shown endogenous methyl groups originating from serine during its conversion to glycine are incorporated into the folate cycle to be transferred to homocysteine for the formation of methionine and ultimately s-adenosyl methionine (SAM), the universal methyl donor. The transfer of methyl groups is catalyzed by several different methyltransferases and result in the formation of s-adenosyl homocysteine (SAH) and methylated products of proteins, lipids, DNA, creatine, phosphotidyl choline etc. SAH is then hydrolyzed to form homocysteine. Homocysteine is the branch point of the methionine cycle, is either methylated back to methionine or participates in the transsulfuration pathway whereby the sulfhydryl group is transferred to serine to form cysteine. Cysteine is a component of the tripeptide, glutathione, the key intracellular antioxidant. The one carbon metabolism requires folate, vitamin B12 (B12), and vitamin B6 (B6). Homocysteine can lead to protein modification, induce inflammation, endothelial damage, thrombosis etc. The formation of SAM from methionine (catalyzed by methionine adenosyl transferase) is sensitive to reactive oxygen injury (ROS) (16-18). Transsulfuration is regulated by change in redox state in addition to by hormones such as insulin and glucagon. Thus at the time of birth both transmethylation and transsulfuration can be effected by the rapid change in redox, by the increased generation of ROS and by the birth associated acute surges in several hormones.
Contributors to Programming(3): Clinical care protocols in the NICU
Evaluation of the contribution of the clinical protocols, used in the neonatal intensive care unit, to programming is confounded by a number of variables, making it difficult to establish cause and effect relationship. The potential variables include:
Heterogeneity of the infant population. As shown in Table 1, infants admitted to the NICU represent a wide range of gestational age and therefore development. These data also show that, over the last twenty years, the relative proportion of various gestation groups and their survival rate has not changed significantly. Since the potential for vulnerability to environmental and other insults may be different at different stages of development, these data underscore the need to examine these gestation groups separately when examining the possible programming effects.
The clinical intervention for cardio-respiratory support, for treatment of sepsis, fluid therapy, and other problems related to prematurity such as hyperbilirubinemia, necrotizing enterocolitis, patent ductus arteriosus, hypoglycemia, anemia, could have added modifying influences. In addition, these interventions can be institution specific depending on the local expertise and bias and are not easily quantifiable in data analysis.
The protocols for clinical care have continuously evolved over time by incorporating new innovations including pharmacological, clinical, nutrition and other technologies. In addition certain pharmacological interventions, considered to be useful early on, have been discontinued as the potential harmful effects were recognized. This has been specifically true for the postnatal use of glucocorticoids (dexamethasone) for the prevention and treatment of broncho-pulmonary dysplasia after reports of neuro-developmental impairment were published.
The algorithms of clinical care have other variations introduced by individual care providers based upon the clinical condition of the baby, or the result of individual bias and expertise resulting in the achieved goal being quite different from that planned or desired. These changes in the clinical protocols make it difficult to correlate the observations in the physiological phenotype in adult with the neonatal intervention.
The commonly used clinical interventions in the Neonatal Intensive care unit that could have programming effects on the baby are listed in Table 2. For most of these, the actual cause and effect has not been demonstrated. In the following we will discuss some of the scientific evidence for potential programming and epigenetic effects of these interventions. Additionally, the LBW babies are exposed to environmental factors such as noise (from the isolette and other equipment), light, and other pharmacological interventions like caffeine, oxygen, etc. Their effects, if any, in relation to programming have not been determined.
Table 2. Clinical interventions that may have programming effects.
|
Nutritional care in the NICU
Nutrition has been recognized as the major or critical influence in programming during development. Nutrient imbalance at critical periods during development has been suggested to result in long-term programming by causing epigenetic changes in the DNA and by modifications of the histones (22). Over the past many years the protocols and algorithms for the nutritional management of the low birth weight baby have been evolving continuously with the goal of providing enough energy producing substrates and proteins in order to achieve a rate of accretion of lean body mass and body weight that resembles the rate of growth and protein accretion of fetus in-utero. Whether such a goal is sufficient for optimal growth and development, or is it desirable or can it be achieved has been a subject of clinical debate and scientific inquiry for many years and continues to be discussed in the contemporary literature (23). As discussed below these nutritional protocols have resulted in varying rates of growth of the babies in the neonatal period and possible long-term consequences.
Parenteral and Enteral Protein Intake
The quality and quantity of protein, the route (parenteral vs. enteral) of administration and the duration of parenteral nutrition have seen the most dramatic change in the last twenty to thirty years. With the increasing emphasis on early introduction of enteral feeding, due to its recognized clinical benefits, the average duration of parenteral nutrition has decreased remarkably from an average of ~30 to 40 days to the current duration of ~10days in most neonatal intensive care units (11). In addition as the protein requirements of the low birth weight babies were recognized and the potential impact of low protein intake were documented, there has been a greater emphasis on early provision of larger amounts of parenteral amino acids to the low birth weight babies (23,36-30) such that a higher proportion of LBW babies are now given 3-4g/kg.d amino acids starting on day one after birth, with no clinically measurable adverse consequences (28-30). This is in contrast to the clinical practice of twenty and thirty years ago when these infants routinely did not receive amino acids in their parenteral nutrient solutions on the first 2-3 days after birth or received very low quantities (0.5-1.0g/kg.d) often accompanied by low caloric intake in the form of glucose alone.
Low Protein Intake
The impact of low protein intake on long term programming in humans has not been reported in part because the follow up period has been relatively short and because of the confounding effect of other clinical and biological variable in this population. In this context it is important to underscore the differential effect of low protein with low energy intake as compared with low protein intake in the presence of isocaloric energy intake. While the combination of low energy intake along with low protein intake results in metabolic responses akin to starvation i.e. increased rate of lipolysis from the adipose tissue and an increased rate of whole body proteolysis in order to meet the energy requirements, the isocaloric protein deficiency results in markedly different responses. In the rodent, dietary protein restriction during pregnancy results in fetal growth retardation and metabolic programming resulting in hypertension, impaired beta cell function and mass, impaired insulin sensitivity, and other pathological responses in the adult offspring (9). These have been associated with a change in the hypothalamic-pituitary-adrenal axis, changes in renin-angiotensin system, and alterations in the concentrations of catecholamines and adrenoreceptors (9,31). The perturbations in the maternal metabolism that lead to the observed programming responses in the fetus have not been delineated. In the non-pregnant adult rat, isocaloric protein restriction for 7-10 days resulted in significant differential expression of a number of genes involved in cell cycle, cell differentiation, transport, transcription and metabolic processes in the liver (32). The expression of genes involved in fatty acid oxidation and serine biosynthesis was higher while those involved in urea synthesis and fatty acid synthesis was lower in the protein restricted animals. Tracer isotope studies showed that protein restriction caused a 50% increase in de-novo synthesis of serine. Since serine is the primary source of methyl groups for transmethylation in-vivo, it was interesting to note that the measured rates of transmethylation of methionine and the methylation potential (SAM/SAH ratio) was significantly increased in the protein restricted animals. These data were interpreted to suggest a high methylation demand placed on the organism as a result of changes in cell cycle and differentiation. Chronic protein malnutrition in humans identified by low plasma transthyretrin levels was observed to be associated with hyperhomocysteinemia and possibly changes in one carbon metabolism (33). Whether the low birth weight baby responds to dietary protein restriction in a similar manner has not been evaluated. In addition, the long term consequences of such a response or its programming effects, if any, has not been determined. It can only be speculated that isocaloric protein restriction at this critical period of development, potentially, can cause programming effect by changes in the one carbon metabolism, transmethylation and methylation potential. However, the clinical data from earlier studies in the low birth weight babies do not show evidence of isolated low protein intake but show both low protein and energy intake during the first few days after birth (24,34). The early protein energy malnutrition in the NICU has been related to neurodevelomental deficits at age 18 months(35). Perhaps these developmental deficits also should be considered programming affects.
High protein intake
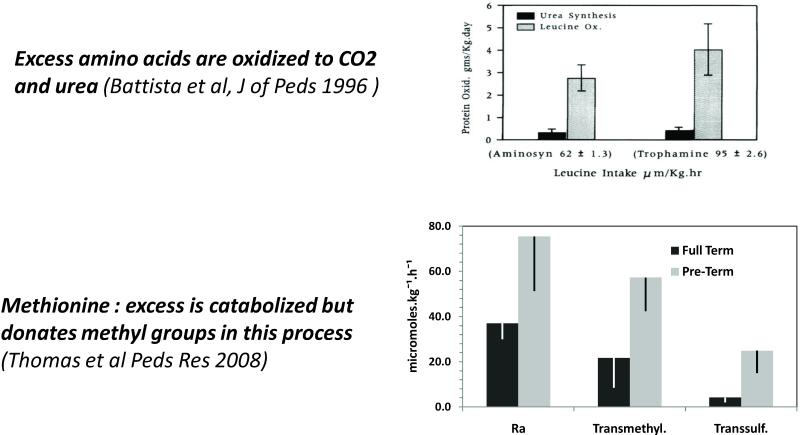
As discussed above, there has been a recent concerted effort to promote higher protein intake by both enteral and parenenteral route and to initiate enteral feedings as early as possible. The higher intake of protein via the enteral route has not been reported to cause any significant problems. In contrast, intravenously administered amino acids mixtures because of their unique composition can cause immediate metabolic changes which may have programming affects. The common amino acid mixture used in the NICU in USA was formulated to result in plasma amino acid concentration resembling that of a term to one month old breastfed infant and does not resemble the amino acid composition of any protein (36). The additional criteria for the formulation were maintenance of solubility and stability of the mixture. In general the commonly used amino acid mixtures (e.g. Trophamine) are high in branched chain amino acids and in methionine and low in cysteine. The early administration of larger (3g/kg.d) amounts of amino acids results in higher plasma concentrations of all amino acids in particular essential amino acids for a variable period without causing any toxic effects (37).Additionally aggressive early administration of higher amount of amino acids did result in positive nitrogen balance and growth of the neonate (28,30). Studies done by Batista and colleagues (30) showed that the majority of excess BCAA in Trophamine are oxidized to CO2 and urea and are not retained in body proteins (Figure 2). Similarly essential amino acid methionine, also administered in larger quantities than its estimated daily requirement (~28 micromoles per kg.h as compared with the estimated requirement of 16 micromoles/kg.h) is also transsulfurated and oxidized to CO2 (39). However for it to enter transsulfuration pathway, the excess methionine enters the transmethylation cycle, and is converted to SAM, SAH and homocysteine (Figure 1). The higher transmethylation will result in an excess methyl load on the organism. While the consequence of the excess methyl load on the low birth weight baby are not known, data from animal models show that excess methyl load during pregnancy in mice can lead to the development of allergic asthma in the offspring(40). Corresponding data in humans have been inconsistent (41,42). However it is significant to note that bronchial asthma has consistently been identified as a significant morbidity at followup in low birth weight babies in several studies (3,5). The contribution of excess methyl load in the parenteral nutrition on long term programming requires careful longitudinal follow up studies.
Fig. 2.
Metabolism of leucine and methionine in preterm infants receiving parenteral amino acid nutrition. (upper panel) The effect of branched amino acid enriched parenteral nutrition (Trophamine) on rates of leucine oxidation and urea synthesis in preterm babies. The rates of leucine oxidation were measured using tracer [1-13C]leucine tracer and the rate of synthesis of urea using [15N2]urea tracer. As shown excess BCAA were oxidized to CO2 and urea. (lower panel) Rate of appearance of methionine (Ra) in the plasma and estimates of transmethylation and transsulfuration in healthy full term babies and preterm babies receiving parenteral amino acid nutrition. As shown the parenteral amino acid containing higher than required methionine (high Ra) resulted in increased transmethylation and transsulfuration.
Growth from “Birth to Term”
Data reported by a number of investigators from clinical centers across the world have underscored the difficulties in achieving the goal of growth in the neonatal period that will mimic the rate of intrauterine growth. These studies show that in spite of our best efforts in clinical and nutritional care of the low birth weight baby, these babies at the time of discharge from the hospital do not achieve the median birth weight of the reference fetus at the same postmenstrual age(11,24,25). Although early and aggressive parenteral and enteral nutritional strategies have been shown to attenuate this postnatal growth failure, the goal of achieving intrauterine rate of growth remains allusive (25,34,43,44). The postnatal growth retardation or the so called “birth to term” growth failure has been described as an inevitable and universal problem in preterm infants (34). The potential contributors to the birth to term growth failure, in addition to nutritional support, include neonatal morbidities such as lung disease, necrotizing enterocolitis, intraventricular hemorrhage, treatment with glucocorticoids etc. Statistical model analysis of the various contributors to the postnatal growth showed that medical morbidities and nutritional factors, in particular protein intake, explained ~50% of the variance in the overall growth velocity (24). Body composition studies of these infants using dual emission X-ray absorptiometry (DEXA) showed reduced linear growth and reduced fat free mass coupled with increased global and central fat mass (45). Whether such body composition changes at this early stage in life have any relation to the development of insulin resistance and metabolic syndrome later in life remains to be examined. Comparison with the studies from India of “fat thin baby” would suggest a high risk of development of insulin resistance later in life (46). A significant relationship between growth velocity in the neonatal intensive care unit and the likelihood of neurodevelopmental impairment has also been reported (35,47). Whether the impaired neurodevelopment is the consequence of programming or due to an acute irreversible injury to the brain is not clear.
Phrmacological Interventions
A number of pharmacological agents, commonly used in the NICU, are listed in Table 2. Data on their long term consequence are limited. Some of these agents such as caffeine, surfactant are administered to almost all low birth weight babies and therefore their consequences are difficult to delineate. Others like nitric oxide, erythropoietin, VEGF inhibitors have only recently been introduced in clinical care and therefore no long term follow up data are available as yet. In the following available data on some of these are discussed in the context of their potential role in programming.
Vitamin A
The plasma concentrations of retinol and retinol binding protein levels in low birth weight babies have been observed to be low in a number of studies. These low levels do not appear to be influenced by the amount and route of feeding and remain low for several months after discharge from the hospital (48-51). These data have been interpreted to represent a state of vitamin A deficiency. Data from studies in-vivo and in-vitro have demonstrated a critical role of vitamin A in the differentiation of pulmonary cells, in surfactant protein metabolism, in airway growth, and expression of several genes involved in the development of lungs. In addition clinical observation studies had suggested that poor vitamin A status in the first months after birth in low birth weight babies was associated with an increased risk of developing bronchopulmonary dysplasia and long-term respiratory disability (51,52). Based upon these data, clinical trials of oral or parenteral vitamin A supplement have been done. These studies show that administration of vitamin A during the first four weeks after birth results in significant but small reduction in death or oxygen use at 36 weeks postmenstrual age (53-56)). Even though the clinical impact of vitamin A supplement was small, it was recommended that 5000IU of vitaminA be given intramuscularly to low birth weight babies who are at risk for developing chronic lung disease or brochopulmonary dysplasia. However such a recommendation is not uniformly followed by the clinical neonatologists(57,58).
The long term consequences and potentially programming effects of the administration of vitamin A to the LBW infants have not been delineated. Limited clinical data of relatively short follow up period suggests that there are no identifiable acute toxicity of high dose vitamin A in the neonate. In addition no significant impact on mortality and neuro-developmental impairment at age 18 to 22 months was observed (56,59). However high dose vitamin A could potentially have programming effect by modulating SAM dependent transmethylation reactions (Figure 1). Studies in rat show that high dose vitamin A and its derivatives (all-trans retinoic acid) caused an increase in abundance and activity of glycine n-methytransferase (GNMT) and hypomethylation of DNA in the liver (60-63)). Interestingly the observed responses were both tissue and gender specific, so that there was no effect of retinoids on pancreatic and renal GNMT activity (62). In addition the increase in heapatic GNMT activity was less in female rats when compared with the males. The long term phenotypic effect of these changes in methylation have not been reported. However exposure of rats to very high (80,000IU/kg/day) doses of vitamin A during 1-5 days after birth has been shown to result in long-lasting (at age 36 weeks) defect in learning in water-maze task and depression in heat-pain responses(64). Other data suggestive of programming effect on immune system have been reported in response to neonatal exposure to vitamin A in rats (65). A pattern of motor deficit following late embryonic exposure to all -trans retinoic acid has also been reported (66). Future studies will examine whether these observations are due to a programming effect related to changes in methylation status caused by perturbation in methionine cycle.
Glucocorticoids
Glucocorticoids have been and remain the most extensively used pharmacological intervention used in the perinatal period. Data from animal models have clearly demonstrated the programing effect of glucocorticoids administration during development. These studies show that exposure to excess glucocorticoids in the prenatal period either due to increased endogenous production as a result of maternal stress, or by exogenous administration, or as a result of inhibition of 11-β hydroxysteroid dehydrogenase type 2 (the placental barrier to maternal glucocorticoids) results in lower birth weight and causes hyperglycemia, hypertension, increased HPA axis activity and behavior abnormalities in the offspring (67,68). These effects have been observed to be transmitted across generations suggesting an epigenetic mechanism. It should be underscored that while animal models are critical for our understanding of the biological and molecular mechanisms of programming, the doses of pharmacological agents used and the duration of administration in these studies have been quite different than those used in clinical practice. In clinical practice, glucocorticoids are either used antenataly to induce pulmonary surfactant synthesis in case of impending premature delivery or in the neonate with respiratory distress syndrome or bronchopulmonary dysplasia in order to wean them from respiratory/ventilator support and minimize the risk of lung injury.
Antenatal Glucocorticoids
A single course of glucocrticoids, either betamethasone or dexamethasone, given to the mother has been shown to be effective in reducing morbidity and mortality after premature birth with few immediate side effects. Both of these glucocrticoids are transferred to the fetus in significant amounts without being metabolized in the placenta. Clinical observation studies have not demonstrated any significant biological impact (programming) on the offspring of this single course of steroid administration, although certain subtle and inconsistent effects have been reported. For example Doyle and colleagues observed a significantly higher systolic and diastolic blood pressure at 14 years of age in subjects exposed to antenatal corticosteroids(69). In contrast Dessens and colleagues observed significantly lower systolic blood pressure at 20 to 22years of age in subjects exposed to antenatal betamethasone (70). Long term follow up studies from different parts of the world have not shown any impact of a single course of antepartum glucocorticoids on body composition, insulin resistance, lipid profile, or other medical or psychological variables (71-74). Finken and colleagues(71) had observed a lower glomerular filteration rate at 19 years of age, while Dalziel et al (72) observed higher plasma insulin levels at 30 minutes following a 75g oral glucose tolerance test at age 30 years. These later observations require confirmation, by additional follow up studies. Exposure to a repeat dose of betamethasone during the antepartum period was also not associated with anthropometric or neurcognitive changes, however the follow up period of these studies was only 2 to 3 years (75,76).
Neonatal Glucocorticoids
The data regarding neonatal use of steroids and their programming effects are confounded by the inconsistencies of dosage regimen and follow up. These data show that postnatal dexamethasone leads to improved ventilation and facilitates weaning from the ventilator support irrespective of dose and timing of administration. As anticipated the use of glucocorticoids in the neonate is associated with variable increase in blood glucose and insulin concentrations, impaired weight gain, clinical complications such as necrotizing enterocolitis etc (79). Follow up data suggest adverse neurological side effects with their use (77-79). Neonatal dexamethasone treatment in rats has been shown to cause sustained lung hypoplasia and increased pulmonary arterial pressure in adult rats (80).
Nitric Oxide
Nitric oxide, a major endogenous regulator of vascular tone (endothelial vascular relaxing factor) and intracellular signaling molecule, is being used extensively for the treatment of pulmonary hypertension in the neonates born at term or at near-term gestation (81). Systematic reviews and meta analysis of the published literature have confirmed the effectiveness of inhaled NO in reducing death and the need for ECMO (extracorporeal membrane oxygenation) without any measurable systemic toxicity in the doses employed (81). Although not approved for use in the prematurely born babies, it is often used outside the licensed indication (off-label) in preterm babies for the prevention and treatment of broncho-pulmonary dysplasia. The rational for the use of inhaled nitric oxide in the prematurely born infants, and its clinical effectiveness (or lack of it) have been discussed (82-84). The potential acute toxic effects of inhaled NO include acute lung injury by peroxynitrite formed by the reaction of NO with oxygen, impaired platelet aggregation, methemoglobinemia and possibly extrapulmonary vasodilatation (85). Nitric oxide is rapidly inactivated by hemoglobin to form methemoglobin. The extrapulmonary effects of inhaled nitric oxide have been attributed to its binding to circulating albumin or hemoglobin and thus delivery of nitric oxide in its active form to systemic circulation (85). In addition nitric oxide is also considered to be an epigenetic molecule (86). Nitric oxide exerts its effect on gene expression and regulation via nitrosylation and tyrosine (tyr)-nitration of a number of nuclear and non-nuclear proteins (86). The epigenetic effects of NO have particularly been studied in the developing neurons (87). Although not examined in relation to therapeutic doses of NO used in the premature infants, the transfer of NO as protein bound adduct (88) could potentially allow for its transport to distant organs and consequently result in epigenetic modification of gene expression. Carefully conducted studies in-vivo and in-vitro would delineate such effects in the future.
Anti-VEGF therapy
Bevacizumab (Avastin), an anti-VEGF therapy, previously used to treat diabetic retinopathy and macular degeneration in adults is now being implemented in preterm NICU infants between 30 and 40 weeks post menstrual age for the treatment of retinopathy of prematurity. It is a recombinant humanized vascular endothelial VEGF antibody that prevents VEGF from binding to its receptors. (89) Bevacizumab binds to all isoforms of VEGF (90), blocks VEGF induced angiogenesis and is approved by the US Food and Drug Administration for intravenous treatment of metastatic colon cancer. A recent randomized controlled trial of intravitreal injection of bevacizumab for stage3+ retinopathy of prematurity in the US has shown lower rates of recurrence of retinopathy (anti-VEGF: 6% vs. laser: 42%), rates of macular dragging (3% vs. 48%) and retinal detachment (0% vs. 6%) in the bevacizumab group compared to the laser therapy group. (91) Although seemingly an exciting new therapy with great potential to minimize serious sequelae of prematurity, information on the long term implications of this therapy remains sparse. Intravitreal bevacizumab enters the general circulation, results in prolonged VEGF inhibition and has a half-life of up to two weeks in primates. In the fetus, VEGF is expressed in most tissues and is critical for growth and development of vital organs such as kidneys, lungs and brain during the third trimester. In-vitro studies on the effect of bevacizumab-induced inhibition of VEGF have demonstrated dose-dependent inhibition of human umbilical vein endothelial cell proliferation (92), increased mortality and impaired liver and cartilage growth in newborn mice (93, 94) and measurable levels of anti-VEGF drug in kidneys, spleen, lung, opposite eye and brain of newborn rabbits receiving intravitreal injection in a single eye.(95) Preterm infants with ROP have a compromised blood-retinal barrier that may allow even more bevacizumab to enter the blood stream. Given the fact that preterm infants receive treatment for ROP at a time when growth and differentiation is maximal, the potential of such a therapy on programming requires careful evaluation.
Others
Bisphenol-A (BPA), an estrogen-mimic endocrine disrupter, is extensively used in manufacturing polycarbonate plastic and epoxy resins from which food and beverage containers and dental materials are made (96,97). BPA may also be used in polyvinyl chloride (PVC) industry. PVC is used in the manufacture of several medical products used in the NICU such as feeding tubes, umbilical vessel catheters, bags and tubings for parenteral fluids and for their administration, endotracheal tubes, respiratory masks etc. Di(2-ethylhexyl) phthalate is a plasticizer added to PVC in a number of these medical products to make them soft and pliable. Calafat and colleagues (97) in a small study examined the exposure to BPA and other phenols in premature infants in two neonatal intensive care units in Massachusetts, USA. Their data showed that the geometric mean BPA concentration among premature infants undergoing intensive medical interventions was one order of magnitude higher than that seen in the general population. Additionally they found that the conjugated species of phenols were the primary urinary metabolites suggesting that the premature LBW babies had some capacity to metabolize BPA. Whether such an exposure to BPA in the LBW babies has any programming effects continues to be an important area of investigation. Data from studies in animal models show that exposure to BPA during early development can change the phenotype of the offspring by stably altering the epigenome(98). Interestingly administration of methyl donors such as folic acid negated the DNA hypomethylating effect of BPA in this study. Other studies have shown disruptive effects of BPA on the expression of sexually selected traits in adults as a consequence of developmental exposure in the mouse and alterations in the development of mammary gland in the rhesus monkey (99).
Contributors to Programming(4): Catch up and Catch down growth during infancy
With the improvement in nutritional care of the preterm babies after discharge from the hospital the frequency of growth failure or stunting has markedly decreased and most prematurely born low birth weight babies show evidence of catch up growth (100-102). However, weight gain alone should not be considered evidence of catch up growth. Uthaya and colleagues studied the body composition of preterm low birth weight babies (<32 weeks gestation) using whole body MRI technique (103). Their data show that at the postconceptional age of term gestation, the preterm babies had significant decrease in subcutaneous adipose tissue and significant increase in visceral adipose tissue while accelerated weight gain in these babies was accompanied by increased total and subcutaneous adiposity. They suggested that preterm baby may be at risk in later life of metabolic complications through increased and aberrant adiposity. However a study by Cooke and colleagues of preterm infants fed a nutrient enriched formula did not find evidence of altered adiposity measured longitudinally during first six months using DEXA method (104). DEXA does not differentiate subcutaneous from visceral fat. A number of other studies, reviews and systematic analysis have examined the impact of after hospital discharge nutrient management of low birth weight babies and catch up and catch down growth(105-107). In the majority of the studies low birth weight does not represent the low birth weight graduate of the NICU but rather small for gestational age full term babies (105-107). Outcome measurements in these infants are confounded by their response to the adverse intrauterine environment. Additionally catch up growth in the low birth weight preterm babies has been observed to continue until age 6 years (108). Accelerated growth or rapid weight gain has some distinct clinical advantages related to decrease in infections and improved rate of survival (105). However, it also has been consistently related to the development of obesity in later life in both appropriate for gestational age and small for gestational age full term and preterm infants (109,110). A detailed analysis of these data are beyond the scope of this review and the reader is referred to some excellent publications on the subject. Obesity, in particular visceral adiposity, is associated with the insulin resistance and metabolic syndrome.
Clinical Outcomes and their correlates
Follow up data show that the low birth weight babies have a higher incidence of hypertension, visceral obesity, asthma and neuro-developmental and behavior problems and perturbations in glucose insulin homeostasis. Whether these non-communicable disorders are the consequence of programming as a result on events during development has been examined only in the case of insulin resistance. These analyses are by necessity statistical correlations of observational data and do not represent causality. The reported data on insulin resistance are discussed below.
Insulin Sensitivity and Glucose Metabolism
The association between insulin sensitivity, altered glucose metabolism at different ages and premature birth (low birth weight or LBW) has been reported in a number of studies from different parts of the world. Hofman and colleagues (111) examined the parameters of glucose metabolism in 38 prepubertal children age 4 to 10 years, born prematurely at less than 32 week gestation and compared them with 22 control subjects (appropriate for gestation age and born after 37 week gestation). Insulin sensitivity was measured by the use of paired glucose insulin data obtained by frequent sampling intravenous glucose tolerance test. Their data show that children born prematurely had a lower insulin sensitivity compared with the controls. In addition the prematurely born subjects had elevated acute insulin release compared with the controls. An altered glucose metabolism (negative correlation between 30 minute glucose concentration post standardized glucose load and birth weight) was also observed by Fewtrell et al (112) in children born prematurely and studies between 9 and 12 years of age. Perturbations in glucose and insulin levels and measures of insulin sensitivity have been demonstrated at ages 18-27yrs using 75g oral GTT and HOMA-R (113), and at age 19yrs using HOMA (114), at age 22yrs using hyperinsulinemic clamp technique (115). All these studies show lower insulin sensitivity in adults born prematurely in different parts of the world (105-115). In addition an association between low birth weight and type 2 diabetes has also been observed in a random sample of middle aged (30 to 60 yrs old) Danes (96).
Whether intrauterine growth restriction is related to insulin sensitivity was also examined by Hofman and colleagues (89). Interestingly intrauterine growth restriction or small for gestational age did not have any additional effect on the change in insulin sensitivity in the premature group (111). Similarly no effect of intrauterine growth restriction on parameters of glucose and insulin metabolism during a standard 75g glucose tolerance test in young adults at 18 to 27years of age (113) and by Rotteveel et al (115) in non obese young adults age 22yrs who were born prematurely at <32 weeks gestation and weighing <1500gms. Finally data from a random sample of middle aged Danes showed a similar prevalence of type 2 diabetes in subjects born prematurely and those born small for gestational age (116) Similar data have been reported by others (117)
Effect of “from birth to term” growth failure: In spite of the best clinical and nutritional practices, most LBW infants at the time of discharge are small for their postmenstrual age(11). The effect of growth failure from birth to term (40 weeks) on insulin sensitivity was reported in two studies.. Fewtrell and colleagues (112) did not observe any relation between growth during the immediate postnatal period (but before term) and glucose, insulin responses at age 9-12 years. Similarly neither a decrease in SD scores for weight at 40 weeks of postmenstrual age (−1.4 +/− 1.32) nor the actual SD score at term (−2.6 =/− 1.15) were related to glucose or insulin concentration, nor was either related to HOMA-IR index at age 18-27 years (113). However in a group of prematurely born SGA infants, the change in SD scores for weight from birth to term was −0.2 =/−0.69; in this group an increase of 1 SD unit in the score corresponded to a 30.8% increase in fasting insulin concentration and 22.8% increase in the 2 h insulin concentration following a standardized 75g glucose load. These data suggest that the growth failure from birth to term menstrual or post conceptional age does not appear to influence development of insulin resistance in adult life in the prematurely born appropriate for gestational age babies. In contrast there is some suggestion that weight gain in small for gestational age baby is related to greater insulin responses later. These data require confirmation in specifically designed studies. Child hood weight gain after 18months of age was the primary determinant of fasting split proinsulin and post standardized glucose load insulin concentration.
These data suggest that interruption of pregnancy i.e. premature delivery and related events independent of IUGR, neonatal clinical support, and growth restriction, is the primary determinant of lower insulin sensitivity later in life. It is compounded by accelerated weight gain (catch up) in infancy. Although the mechanism or mechanisms responsible for the pancreatic insult are not known, we hypothesize that they may be related to the change in redox or to the exposure of the developing beta cell to parenteral and enteral nutrients administered to the LBW infant to support extrauterine survival.
Acknowledgements
The authors thank Susan Marczewski RN and Manoa Hui for their help in the preparation of this manuscript. The cited work was supported in part by NIH grant HD042152 and by Clinical and Translational Science grant award (RR024989) to Case Western Reserve University.
We apologize to those authors whose important work, we may not have cited or omitted inadvertently due to space constraints.
References
- 1.Eichenwald EC, Stark AR. Management and outcomes of very low birth weight. N Engl J Med. 2008 Apr 17;358(16):1700–11. doi: 10.1056/NEJMra0707601. [DOI] [PubMed] [Google Scholar]
- 2.Fanaroff AA, Hack M, Walsh MC. The NICHD neonatal research network: Changes in practice and outcomes during the first 15 years. Semin Perinatol. 2003 Aug;27(4):281–7. doi: 10.1016/s0146-0005(03)00055-7. [DOI] [PubMed] [Google Scholar]
- 3.Hack M. Adult outcomes of preterm children. J Dev Behav Pediatr. 2009 Oct;30(5):460–70. doi: 10.1097/DBP.0b013e3181ba0fba. [DOI] [PubMed] [Google Scholar]
- 4.Wilson-Costello D, Friedman H, Minich N, Fanaroff AA, Hack M. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics. 2005 Apr;115(4):997–1003. doi: 10.1542/peds.2004-0221. [DOI] [PubMed] [Google Scholar]
- 5.Doyle LW, Anderson PJ. Adult outcome of extremely preterm infants. Pediatrics. 2010 Aug;126(2):342–51. doi: 10.1542/peds.2010-0710. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson J, Forsen T, Tuomilehto J, Osmond C, Barker D. Fetal and childhood growth and hypertension in adult life. Hypertension. 2000 Nov;36(5):790–4. doi: 10.1161/01.hyp.36.5.790. [DOI] [PubMed] [Google Scholar]
- 7.Hovi P, Andersson S, Raikkonen K, Strang-Karlsson S, Jarvenpaa AL, Eriksson JG, Pesonen AK, Heinonen K, Pyhala R, Kajantie E. Ambulatory blood pressure in young adults with very low birth weight. J Pediatr. 2010 Jan;156(1):54–59.e1. doi: 10.1016/j.jpeds.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Whincup PH, Kaye SJ, Owen CG, Huxley R, Cook DG, Anazawa S, Barrett-Connor E, Bhargava SK, Birgisdottir BE, Carlsson S, et al. Birth weight and risk of type 2 diabetes: A systematic review. JAMA. 2008 Dec 24;300(24):2886–97. doi: 10.1001/jama.2008.886. [DOI] [PubMed] [Google Scholar]
- 9.Warner MJ, Ozanne SE. Mechanisms involved in the developmental programming of adulthood disease. Biochem J. 2010 Apr 14;427(3):333–47. doi: 10.1042/BJ20091861. [DOI] [PubMed] [Google Scholar]
- 10.Simmons R. Developmental origins of adult metabolic disease: Concepts and controversies. Trends Endocrinol Metab. 2005 Oct;16(8):390–4. doi: 10.1016/j.tem.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Ehrenkranz RA, Younes N, Lemons JA, et al. Longitudinal growth of hospitalized very low birth weight infants. Pediatrics. 1999;104:280–9. doi: 10.1542/peds.104.2.280. [DOI] [PubMed] [Google Scholar]
- 12.Philippidis H, Ballard FJ. The development of gluconeogenesis in rat liver: Experiments in vivo. Biochem J. 1969 Jul;113(4):651–7. doi: 10.1042/bj1130651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filippi L, Messeri A, Dani C, Pezzati M, Tronchin M, Giani T, Bossoli S, Rubaltelli FF. Redox status in very-low birth-weight newborns. Biol Neonate. 2004;85(3):210–6. doi: 10.1159/000075834. [DOI] [PubMed] [Google Scholar]
- 14.Avery SV. Molecular targets of oxidative stress. Biochem J. 2011 Mar 1;434(2):201–10. doi: 10.1042/BJ20101695. [DOI] [PubMed] [Google Scholar]
- 15.Kalhan SC, Marczewski SE. Methionine, Homocysteine, One Carbon Metabolism and Fetal Growth. Rev Endocr Metab Disord. 2012;13(2):109–19. doi: 10.1007/s11154-012-9215-7. [DOI] [PubMed] [Google Scholar]
- 16.Prudova A, Bauman Z, Braun A, Vitvitsky V, Lu SC, Banerjee R. S-adenosylmethionine stabilizes cystathionine β synthase and modulates redox capacity. Proc Nat Acad Sci. 2006;103:6489–6494. doi: 10.1073/pnas.0509531103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vivitsky V, Mosharov E, Tritt M, Ataullakhanov F, Banerjee R. Redox regulation of homocysteine-dependent glutathione synthesis. Redox Report. 2003;8(11):57–63. doi: 10.1179/135100003125001260. [DOI] [PubMed] [Google Scholar]
- 18.Kabil O, Banerjee R. Redox biochemistry of hydrogen sulfide. J Biol Chem. 2010;285:21903–7. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalhan S, Parimi P. Gluconeogenesis in the fetus and neonate. Semin Perinatol. 2000 Apr;24(2):94–106. doi: 10.1053/sp.2000.6360. [DOI] [PubMed] [Google Scholar]
- 20.Kalhan SC, Bier DM. Protein and amino acid metabolism in the human newborn. Annu Rev Nutr. 2008;28:389–410. doi: 10.1146/annurev.nutr.28.061807.155333. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasan M, Laychock SG, Hill DJ, Patel MS. Neonatal nutrition: Metabolic programming of pancreatic islets and obesity. Exp Biol Med (Maywood) 2003 Jan;228(1):15–23. doi: 10.1177/153537020322800102. [DOI] [PubMed] [Google Scholar]
- 22.Symonds ME, Sebert SP, Hyatt MA, Budge H. Nutritional programming of the metabolic syndrome. Nat Rev Endocrinol. 2009 Nov;5(11):604–10. doi: 10.1038/nrendo.2009.195. [DOI] [PubMed] [Google Scholar]
- 23.Hay WW, Thureen P. Protein for preterm infants: How much is needed? how much is enough? how much is too much? Pediatr Neonatol. 2010 Aug;51(4):198–207. doi: 10.1016/S1875-9572(10)60039-3. [DOI] [PubMed] [Google Scholar]
- 24.Olsen IE, Richardson DK, Schmid CH, Ausman LM, Dwyer JT. Intersite differences in weight growth velocity of extremely premature infants. Pediatrics. 2002 Dec;110(6):1125–32. doi: 10.1542/peds.110.6.1125. [DOI] [PubMed] [Google Scholar]
- 25.Cooke RJ, Ainsworth SB, Fenton AC. Postnatal growth retardation: A universal problem in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2004 Sep;89(5):F428–30. doi: 10.1136/adc.2001.004044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hay WW, Jr, Thureen PJ. Early postnatal administration of intravenous amino acids to preterm, extremely low birth weight infants. J Pediatr. 2006 Mar;148(3):291–4. doi: 10.1016/j.jpeds.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Poindexter BB, Langer JC, Dusick AM, Ehrenkranz RA. National Institute of Child Health and Human Development Neonatal Research Network. Early provision of parenteral amino acids in extremely low birth weight infants: Relation to growth and neurodevelopmental outcome. J Pediatr. 2006 Mar;148(3):300–5. doi: 10.1016/j.jpeds.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 28.te Braake FW, van den Akker CH, Wattimena DJ, Huijmans JG, van Goudoever JB. Amino acid administration to premature infants directly after birth. J Pediatr. 2005 Oct;147(4):457–61. doi: 10.1016/j.jpeds.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 29.Jadhav P, Parimi PS, Kalhan SC. Parenteral amino acid and metabolic acidosis in premature infants. JPEN J Parenter. Enteral Nutr. 2007 Jul-Aug;31(4):278–83. doi: 10.1177/0148607107031004278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibrahim HM, Jeroudi MA, Baier RJ, Dhanireddy R, Krouskop RW. Aggressive early total parental nutrition in low-birth-weight infants. J Perinatol. 2004 Aug;24(8):482–6. doi: 10.1038/sj.jp.7211114. [DOI] [PubMed] [Google Scholar]
- 31.Augustyniak RA, Singh K, Zeldes D, Singh M, Rossi NF. Maternal protein restriction leads to hyperresponsiveness to stress and salt-sensitive hypertension in male offspring. Am J Physiol Regul Integr Comp Physiol. 2010 May;298(5):R1375–82. doi: 10.1152/ajpregu.00848.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalhan SC, Uppal SO, Moorman JL, Bennett C, Gruca LL, Parimi PS, Dasarathy S, Serre D, Hanson RW. Metabolic and genomic response to dietary isocaloric protein restriction in the rat. J Biol Chem. 2011 Feb 18;286(7):5266–77. doi: 10.1074/jbc.M110.185991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ingenbleek Y, Hardillier E, Jung L. Subclinical protein malnutrition is a determinant of hyperhomocysteinemia. Nutrition. 2002 Jan;18(1):40–6. doi: 10.1016/s0899-9007(01)00783-3. [DOI] [PubMed] [Google Scholar]
- 34.Embleton NE, Pang N, Cooke RJ. Postnatal malnutrition and growth retardation: An inevitable consequence of current recommendations in preterm infants? Pediatrics. 2001 Feb;107(2):270–3. doi: 10.1542/peds.107.2.270. [DOI] [PubMed] [Google Scholar]
- 35.Stephens BE, Walden RV, Gargus RA, et al. First-week protein and energy intakes are associated with 18-month developmental outcomes in extremely low birth weight infants. Pediatrics. 2009;123:1337–43. doi: 10.1542/peds.2008-0211. [DOI] [PubMed] [Google Scholar]
- 36.Heird WC, Hay W, Helms RA, Storm MC, Kashyap S, Dell RB. Pediatric parenteral amino acid mixture in low birth weight infants. Pediatrics. 1988;81:41–50. [PubMed] [Google Scholar]
- 37.Blanco CL, Gong AK, Green BK, Falck A, Schoolfield J, Liechty EA. Early changes in plasma amino acid concentrations during aggressive nutrition therapy in extremely low birth weight infants. J Pediatr. 2011;158:543–8. doi: 10.1016/j.jpeds.2010.09.082. [DOI] [PubMed] [Google Scholar]
- 38.Battista MA, Price PT, Kalhan SC. Effect of parenteral amino acids on leucine and urea kinetics in preterm infants. J Pediatr. 1996;128:130–140. doi: 10.1016/s0022-3476(96)70442-0. [DOI] [PubMed] [Google Scholar]
- 39.Thomas B, Gruca LL, Bennett C, Parimi PS, Hanson RW, Kalhan SC. Metabolism of methionine in the newborn infant: response to the parenteral and enteral administration of nutrients. Pediatr Res. 2008;64:381–386. doi: 10.1203/PDR.0b013e318180e499. PMCID: 2651408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hollingsworth JW, Maruoka S, Boon K, Garantziotis S, Li Z, Tomfohr J, Bailey N, Potts EN, Whitehead G, Brass DM, Schwartz DA. In utero supplementation with methyl donors enhances allergic airway disease in mice. Journal of Clin Invest. 2008;118:3462–69. doi: 10.1172/JCI34378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Haberg SE, London SJ, Stigum H, Nafstad P, Nystad W. Folic acid supplements in pregnancy and early childhood respiratory health. Arch Dis Child. 2009;94:180–184. doi: 10.1136/adc.2008.142448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whitrow MJ, Moore VM, Rumbold AR, Davies MJ. Effect of supplemental folic acid in pregnancy on childhood asthma: a prospective birth cohort study. Am J Epidemiol. 2009;170:1486–93. doi: 10.1093/aje/kwp315. [DOI] [PubMed] [Google Scholar]
- 43.Wilson DC, Cairns P, Halliday HL, Reid M, McClure G, Dodge JA. Randomised controlled trial of an aggressive nutritional regimen in sick very low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 1997 Jul;77(1):F4–11. doi: 10.1136/fn.77.1.f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dinerstein A, Nieto RM, Solana CL, Perez GP, Otheguy LE, Larguia AM. Early and aggressive nutritional strategy (parenteral and enteral) decreases postnatal growth failure in very low birth weight infants. J Perinatol. 2006 Jul;26(7):436–42. doi: 10.1038/sj.jp.7211539. [DOI] [PubMed] [Google Scholar]
- 45.Cooke RJ, Griffin I. Altered body composition in preterm infants at hospital discharge. Altered body composition in preterm infants at hospital discharge. Acta Paediatr. 2009;98:1269–73. doi: 10.1111/j.1651-2227.2009.01354.x. [DOI] [PubMed] [Google Scholar]
- 46.Yajnik CS, Fall CH, Coyaji KJ, Hirve SS, Rao S, Barker DJ, Joglekar C, Kellingray S. Neonatal anthropometry: The thin-fat indian baby. the pune maternal nutrition study. Int J Obes Relat Metab Disord. 2003 Feb;27(2):173–80. doi: 10.1038/sj.ijo.802219. [DOI] [PubMed] [Google Scholar]
- 47.Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006 Apr;117(4):1253–61. doi: 10.1542/peds.2005-1368. [DOI] [PubMed] [Google Scholar]
- 48.Peeples JM, Carlson SE, Werkman SH, Cooke RJ. Vitamin A status of preterm infants during infancy. Am J Clin Nutr. 1991;53:1455–9. doi: 10.1093/ajcn/53.6.1455. [DOI] [PubMed] [Google Scholar]
- 49.Greene HL, Phillips BL, Franck L, et al. Persistently low blood retinol levels during and after parenteral feeding of very low birth weight infants: examination of losses into intravenous administration sets and a method of prevention by addition to a lipid emulsion. Pediatrics. 1987;79:894–900. [PubMed] [Google Scholar]
- 50.Shenai JP, Chytil F, Stahlman MT. Liver vitamin A reserves of very low birth weight neonates. Pediatr Res. 1985;19:892–3. doi: 10.1203/00006450-198509000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Shenai JP, Chytil F, Stahlman MT. Vitamin A status of neonates with bronchopulmonary dysplasia. Pediatr Res. 1958;19:185–8. doi: 10.1203/00006450-198502000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Spears K, Cheney C, Zerzan J. Low plasma retinol concentrations increase the risk of developing bronchopulmonary dysplasia and long-term respiratory disability in very low birth weight infants. Am J Clin Nutr. 2004;80:1589–94. doi: 10.1093/ajcn/80.6.1589. [DOI] [PubMed] [Google Scholar]
- 53.Shenai JP, Kennedy KA, Chytil F, Stahlman MT. Clinical trial of vitamin A supplementation in infants susceptible to bronchopulmonary dysplasia. J Pediatr. 1987;111:269–77. doi: 10.1016/s0022-3476(87)80086-0. [DOI] [PubMed] [Google Scholar]
- 54.Pearson E, Bose C, Snidow T, et al. Trial of vitamin A supplementation in very low birth weight infants at risk for bronchopulmonary dysplasia. J Pediatr. 1992;121:420–7. doi: 10.1016/s0022-3476(05)81800-1. [DOI] [PubMed] [Google Scholar]
- 55.Wardle SP, Hughes A, Chen S, Shaw NJ. Randomised controlled trial of oral vitamin A supplementation in preterm infants to prevent chronic lung disease. Arch Dis Child Fetal Neonatal Ed. 2001;84:F9–13. doi: 10.1136/fn.84.1.F9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tyson JE, Wright LL, Oh W, et al. Vitamin A supplementation for extremely low birth weight infants. N Engl J Med. 1999;340:1962–8. doi: 10.1056/NEJM199906243402505. [DOI] [PubMed] [Google Scholar]
- 57.Darlow BA, Graham PJ. Vitamin A supplementation to prevent mortality and short and long term morbidity in very low birth weight infants. Cochrane Database Syst Rev. 2011:10. doi: 10.1002/14651858.CD000501.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barros FC, Bhutta ZA, Batra M, et al. Global report on preterm birth and stillbirth (3 of 7): evidence for effectiveness of interventions. BMC Pregnancy Childbirth. 2010;10(Suppl 1):S3. doi: 10.1186/1471-2393-10-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ambalavanan N, Tyson JE, Kennedy KA, et al. Vitamin A supplementation for extremely low birth weight infants: outcome at 18 to 22 months. Pediatrics. 2005;115:e249–54. doi: 10.1542/peds.2004-1812. [DOI] [PubMed] [Google Scholar]
- 60.Rowling MJ, McMullen MH, Schalinske KL. Vitamin A and its derivatives induce hepatic glycine N-methyltransferase and hypomethylation of DNA in rats. J Nutr. 2002;132:365–9. doi: 10.1093/jn/132.3.365. [DOI] [PubMed] [Google Scholar]
- 61.Rowling MJ, Schalinske KL. Retinoid compounds activate and induce hepatic glycine N-methyltransferase in rats. J Nutr. 2001;131:1914–7. doi: 10.1093/jn/131.7.1914. [DOI] [PubMed] [Google Scholar]
- 62.McMullen MH, Rowling MJ, Ozias MK, Schalinske KL. Activation and induction of glycine N-methyltransferase by retinoids are tissue and gender specific. Arch Biochem Biophys. 2002;401:73–80. doi: 10.1016/S0003-9861(02)00030-9. [DOI] [PubMed] [Google Scholar]
- 63.Rowling MJ, Schalinske KL. Retinoic acid and glucocorticoid treatment induce hepatic glycine N-methyltransferase and lower plasma homocysteine concentrations in rats and rat hepatoma cells. J Nutr. 2003;133:3392–8. doi: 10.1093/jn/133.11.3392. [DOI] [PubMed] [Google Scholar]
- 64.Kihara T, Matsuo T, Sakamoto M, Yasuda Y, Tanimura T. Effects of the neonatal vitamin A exposure on behaviors of adult rats. J Toxicol Sci. 1995;20:93–101. doi: 10.2131/jts.20.93. [DOI] [PubMed] [Google Scholar]
- 65.Csaba G, Kovacs P, Pallinger E. Transgenerational effect of neonatal vitamin A or D treatment (hormonal imprinting) on the hormone content of rat immune cells. Horm Metab Res. 2007;39:197–201. doi: 10.1055/s-2007-970418. [DOI] [PubMed] [Google Scholar]
- 66.Coluccia A, Borracci P, Belfiore D, Renna G, Carratu MR. Late embryonic exposure to all trans retinoic acid induces a pattern of motor deficits unrelated to the developmental stage. Neurotoxicology. 2009;30:1120–6. doi: 10.1016/j.neuro.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 67.Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2011;59:279–89. doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 68.Drake AJ, Tang JI, Nyirenda MJ. Mechanisms underlying the role of glucocorticoids in the early life programming of adult disease. Clin Sci (Lond) 2007;113:219–32. doi: 10.1042/CS20070107. [DOI] [PubMed] [Google Scholar]
- 69.Doyle LW, Ford GW, Davis NM, Callanan C. Antenatal corticosteroid therapy and blood pressure at 14 years of age in preterm children. Clin Sci (Lond) 2000;98:137–42. [PubMed] [Google Scholar]
- 70.Dessens AB, Haas HS, Koppe JG. Twenty-year follow up of antenatal corticosteroid treatment. Pediatrics. 2000;105:E77. doi: 10.1542/peds.105.6.e77. [DOI] [PubMed] [Google Scholar]
- 71.Finken MJ, Keijzer-Veen MG, Dekker FW, et al. Antenatal glucocorticoid treatment is not associated with long term metabolic risks in individuals born before 32 weeks of gestation. Arch Dis Child Fetal Neonatal Ed. 2008;93:F442–7. doi: 10.1136/adc.2007.128470. [DOI] [PubMed] [Google Scholar]
- 72.Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30 year follow up of a randomised controlled trial. Lancet. 2005;365:1856–62. doi: 10.1016/S0140-6736(05)66617-2. [DOI] [PubMed] [Google Scholar]
- 73.Dalziel SR, Rea HH, Walker NK, et al. Long term effects of antenatal betamethasone on lung function: 30 year follow up of a randomised controlled trial. Thorax. 2006;61:678–83. doi: 10.1136/thx.2005.051763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dalziel SR, Lim VK, Lambert A, et al. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in randomised controlled trial. BMJ. 2005;331:665. doi: 10.1136/bmj.38576.494363.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wapner RJ, Sorokin Y, Mele L, et al. Long term outcomes after repeat doses of antenatal corticosteroids. N Engl J Med. 2007;357:1190–8. doi: 10.1056/NEJMoa071453. [DOI] [PubMed] [Google Scholar]
- 76.Peltoniemi OM, Kari MA, Lano A, et al. Two year follow up of a randomised trial with repeated antenatal betamethasone. Arch Dis Child Fetal Neonatal Ed. 2009;94:F402–6. doi: 10.1136/adc.2008.150250. [DOI] [PubMed] [Google Scholar]
- 77.Yates H, Newell S. Postnatal intravenous steroids and long term neurological outcome: recommendations from meta analyses. Arch Dis Child Fetal Neonatal Ed. 2011 Mar 22;:F1–5. doi: 10.1136/adc.2010.208868. [DOI] [PubMed] [Google Scholar]
- 78.Wilson-Costello D, Walsh MC, Langer JC, et al. Impact of postnatal corticosteroid use on neurodevelopment at 18 to 22 months’ adjusted age: effects of dose, timing, and risk of bronchopulmonary dysplasia in extremely low birth weight infants. Pediatrics. 2009;123:e430–7. doi: 10.1542/peds.2008-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stark AR, Carlo WA, Tyson JE, et al. Adverse effects of early dexamethasone in extremely low birth weight infants. N Engl J Med. 2001;344:95–101. doi: 10.1056/NEJM200101113440203. [DOI] [PubMed] [Google Scholar]
- 80.le Cras TD, Markhan NE, Morris KG, et al. Neonatal dexamethasone treatment increases the risk for pulmonary hypertension in adult rats. Am J Physiol Lung Cell Mol Physiol. 2000;278:L822–9. doi: 10.1152/ajplung.2000.278.4.L822. [DOI] [PubMed] [Google Scholar]
- 81.Flier NN, Barrington KJ. Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database Syst Rev. 2006;(4):CD000399. doi: 10.1002/14651858.CD000399.pub2. [DOI] [PubMed] [Google Scholar]
- 82.Subhedar N, Dewhurst C. Is nitric oxide effective in preterm infants? Arch Dis Child Fetal and Neonatal ed. 2007;92:337–341. doi: 10.1136/adc.2006.107490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Donohue PK, Gilmore MM, Cristofalo E, Wilson RF, Weiner JZ, Lau BD, Robinson KA, Allen MC. Inhaled nitric oxide in preterm infants: a systematic review. Pediatrics. 2011;127(2):e414–22. doi: 10.1542/peds.2010-3428. [DOI] [PubMed] [Google Scholar]
- 84.Cole FS, Alleyene C, barks JD, Boyle RJ, Carroll JL, Dokken D, Edwards WH, Georgieff M, et al. a. NIH consensus development conference statement: inhaled nitric oxide therapy for premature infants. Pediatrics. 2011;127(2):363–369. doi: 10.1542/peds.2010-3507. [DOI] [PubMed] [Google Scholar]
- 85.Weinberger B, Laskin DL, Heck DE, Laskin JD. The toxicology of inhaled nitric oxide. Toxicological Sciences. 2001;59:5–16. doi: 10.1093/toxsci/59.1.5. [DOI] [PubMed] [Google Scholar]
- 86.Illi B, Colussi C, Grasselli A, Farsetti A, Capgrossi MC, Gaetano C. NO sparks off chromatin: tales of a multifaceted epigenetic regulator. Pharmacology and Therapeutics. 2009;123:344–352. doi: 10.1016/j.pharmthera.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 87.Nott A, Riccio A. Nitric oxide-mediated epigenetic mechanisms in developing neurons. Cell Cycle. 2009;8:725–730. doi: 10.4161/cc.8.5.7805. [DOI] [PubMed] [Google Scholar]
- 88.Keanney JF, Jr, Simon DI, Stamler JS, Jareki O, Scharfstein J, Vita JA, Loscaizo J. NO forms an adduct with serum albumin that has endothelium-derived relaxing factor-like properties. J Clin Invest. 1993;91:1582–89. doi: 10.1172/JCI116364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lin YS, Nguyen C, Mendoza JL, Escandon E, Fei D, Meng YG, et al. Preclinical pharmacokinetics, interspecies scaling and tissue distribution of a humanized monoclonal antibody against vascular endothelial growth factor. J Pharmacol Exp Ther. 1999;288:371–378. [PubMed] [Google Scholar]
- 90.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 91.Mintz-Hittner HA, Kennedy KA, Chuang AZ. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364:603–15. doi: 10.1056/NEJMoa1007374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Y, Fei D, Venderlaan M, Song A. Biological activity of bevacizumab, a humanized anti-VEGF antibody in vitro. Angiogenesis. 2004;7:335–45. doi: 10.1007/s10456-004-8272-2. [DOI] [PubMed] [Google Scholar]
- 93.Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell I, et al. VEGF is required for growth and survival in neonatal mice. Development. 1999;126:1149–59. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- 94.Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623–8. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 95.Wu WC, Lai CC, Chen KJ, Chen TL, Wang NK, Hwang YS, et al. Long-term tolerability and serum concentration of bevacizumab (avastin) when injected in newborn rabbit eyes. Invest Ophthalmol Vis Sci. 2010;51:3701–8. doi: 10.1167/iovs.09-4425. [DOI] [PubMed] [Google Scholar]
- 96.Maffini MV, Rubin BS, Sonnenschein C, Sotoi AM. Endocrine disruptors and reproductive health: The case of bisphenol-A. Molecular and Cellular Endocr. 2006:254–255. 179–186. doi: 10.1016/j.mce.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 97.Calafat AM, Weuve J, Ye X, Jia LT, Hu H, Ringer S, Huttner K, Hauser R. Exposure to Bisphenol A and other phenols in Neonatal Intensive care Unit premature infants. Environ Health Perspect. 2009;117:639–644. doi: 10.1289/ehp.0800265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dolibnoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Nat Acad Sci. 2007;104:13056–61. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jasarevic E, Sieli PT, Twellman EE, Welsh TH, Schachtman TR, Roberts RM, Geary DC, Rosenfeld CS. Disruption of adult expression of sexually selected traits by developmental exposure to bisphenol A. Proc Nat Acad Sci. 2011;108:11715–20. doi: 10.1073/pnas.1107958108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hack M, Merkatz IR, McGrath SK, Jones PK, Fanaroff AA. Catch-up growth in very low birth weight infants. Am J Dis Child. 1984;138:370–5. doi: 10.1001/archpedi.1984.02140420036013. [DOI] [PubMed] [Google Scholar]
- 101.Brandt I, Sticker EJ, Gausche R, Lentze MJ. Catch-up growth of supine length/height of very low birth weight, small for gestational age preterm infants to adulthood. J Pediatr. 2005;147:662–8. doi: 10.1016/j.jpeds.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 102.Itabashi K, Mishina J, Tada H, Sakurai M, Nanri Y, Hirohata Y. Longitudinal follow up of height up to five years of age in infants born preterm small for gestational age; comparison to full term small for gestational age infants. Early Hum Dev. 2007;83:327–33. doi: 10.1016/j.earlhumdev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 103.Uthaya S, Thomas EL, Hamilton G, et al. Altered adiposity after extremely preterm birth. Pediatr Res. 2005;57:211–5. doi: 10.1203/01.PDR.0000148284.58934.1C. [DOI] [PubMed] [Google Scholar]
- 104.Cooke RJ, Griffin IJ, McCormick K. Adiposity is not altered in preterm infants fed with a nutrient enriched formula after hospital discharge. Pediatr Res. 2010;67:660–4. doi: 10.1203/PDR.0b013e3181da8d01. [DOI] [PubMed] [Google Scholar]
- 105.Yeung MY. Postnatal growth, neurodevelopment and altered adiposity after preterm birth— from a clinical nutrition perspective. Acta Paediatr. 2006;95:909–17. doi: 10.1080/08035250600724507. [DOI] [PubMed] [Google Scholar]
- 106.Ong KK, Loos RJ. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr. 2006;95:904–8. doi: 10.1080/08035250600719754. [DOI] [PubMed] [Google Scholar]
- 107.Monteiro PO, Victora CG. Rapid growth in infancy and childhood and obesity in later life—a systematic review. Obes Rev. 2005;6:143–54. doi: 10.1111/j.1467-789X.2005.00183.x. [DOI] [PubMed] [Google Scholar]
- 108.Wehkalampi K, Hovi P, Dunkel L, et al. Advanced pubertal growth spurt in subjects born preterm: the Helsinki study of very low birth weight adults. J Clin Endocrinol Metab. 2011;96:525–33. doi: 10.1210/jc.2010-1523. [DOI] [PubMed] [Google Scholar]
- 109.Bazaes RA, Alegría A, Pittaluga E, et al. Determinants of insulin sensitivity and secretion in very low birth weight children. J Clin endocrinol Metab. 2004;89:1267–72. doi: 10.1210/jc.2003-031239. [DOI] [PubMed] [Google Scholar]
- 110.Stettler N, Iotova V. Early growth patterns and long term obesity risk. Curr Opin Clin Nutr Metab Care. 2010;13:294–9. doi: 10.1097/MCO.0b013e328337d7b9. [DOI] [PubMed] [Google Scholar]
- 111.Hofman PL, Regan F, Jackson WE, Jefferies C, Knight DB, Robinson EM, Cutfield WS. Premature birth and later insulin resistance. N Engl J Med. 2004 Nov 18;351(21):2179–86. doi: 10.1056/NEJMoa042275. Erratum in: N Engl J Med. 2004 Dec 30;351(27):2888. [DOI] [PubMed] [Google Scholar]
- 112.Fewtrell MS, Doherty C, Cole TJ, Stafford M, Hales CN, Lucas A. Effects of size at birth, gestational age and early growth in preterm infants on glucose and insulin concentrations at 9-12 years. Diabetologia. 2000 Jun;43(6):714–7. doi: 10.1007/s001250051368. [DOI] [PubMed] [Google Scholar]
- 113.Hovi P, Andersson S, Eriksson JG, Järvenpää AL, Strang-Karlsson S, Mäkitie O, Kajantie E. Glucose regulation in young adults with very low birth weight. N Engl J Med. 2007 May 17;356(20):2053–63. doi: 10.1056/NEJMoa067187. PubMed PMID: 17507704. [DOI] [PubMed] [Google Scholar]
- 114.Finken MJ, Keijzer-Veen MG, Dekker FW, Frölich M, Hille ET, Romijn JA, Wit JM. Dutch POPS-19 Collaborative Study Group. Preterm birth and later insulin resistance: effects of birth weight and postnatal growth in a population based longitudinal study from birth into adult life. Diabetologia. 2006 Mar;49(3):478–85. doi: 10.1007/s00125-005-0118-y. Epub 2006 Feb 1. [DOI] [PubMed] [Google Scholar]
- 115.Rotteveel J, van Weissenbruch MM, Twisk JW, Delemarre-Van de Waal HA. Infant and childhood growth patterns, insulin sensitivity, and blood pressure in prematurely born young adults. Pediatrics. 2008 Aug;122(2):313–21. doi: 10.1542/peds.2007-2012. [DOI] [PubMed] [Google Scholar]
- 116.Pilgaard K, Færch K, Carstensen B, Poulsen P, Pisinger C, Pedersen O, Witte DR, Hansen T, Jørgensen T, Vaag A. Low birthweight and premature birth are both associated with type 2 diabetes in a random sample of middle-aged Danes. Diabetologia. 2010 Dec;53(12):2526–30. doi: 10.1007/s00125-010-1917-3. Epub 2010 Sep 22. [DOI] [PubMed] [Google Scholar]
- 117.Kaijser M, Bonamy AK, Akre O, Cnattingius S, Granath F, Norman M, Ekbom A. Perinatal risk factors for diabetes in later life. Diabetes. 2009 Mar;58(3):523–6. doi: 10.2337/db08-0558. Epub 2008 Dec 9. [DOI] [PMC free article] [PubMed] [Google Scholar]