Fig. 1.
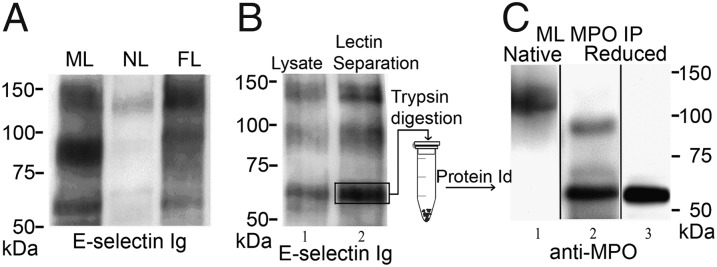
Expression of the ∼65-kDa E-selectin ligand is characteristic of MLs and circulating myeloid cells during leukemoid reactions (FL), and comprises the heavy chain of MPO. (A) Lysates of mobilized leukocytes (MLs), native leukocytes (NLs), and circulating myeloid cells of patients with febrile leukocytosis (FL), normalized for input cell number, were resolved by SDS/PAGE and stained in Western blot with E-selectin Ig chimera (E–Ig). A representative blot of multiple patient samples (n > 14 for each group) is shown. Expression of the ∼65-kDa glycoprotein is characteristic of G-CSF–primed cells (MLs and FLs), but not of NLs. (B) Lysates of MLs (lane 1) and WGA lectin chromatography-purified glycoproteins (lane 2) resolved on reducing 7.5% SDS/PAGE gel and immunoblotted with E-selectin–Ig chimera. E-selectin ligands are present at ∼140 kDa (PSGL-1), ∼100 kDa (HCELL), and at ∼65 kDa. (C) Representative results of Western blots of MPO immunoprecipitates (IPs) from MLs resolved under nonreducing (lane 1) or reducing conditions (lanes 2 and 3) and stained with anti-MPO mAb. In nonreduced gels, the mature homodimer of ∼140 kDa is evident (lane 1). Under reducing conditions, Western blot with anti-MPO mAb 2C7 (lane 2) reveals bands at ∼90 kDa (precursor) and ∼65 kDa (heavy chain) or only the ∼65-kDa band when stained with anti-MPO mAb 3D3 (lane 3).
