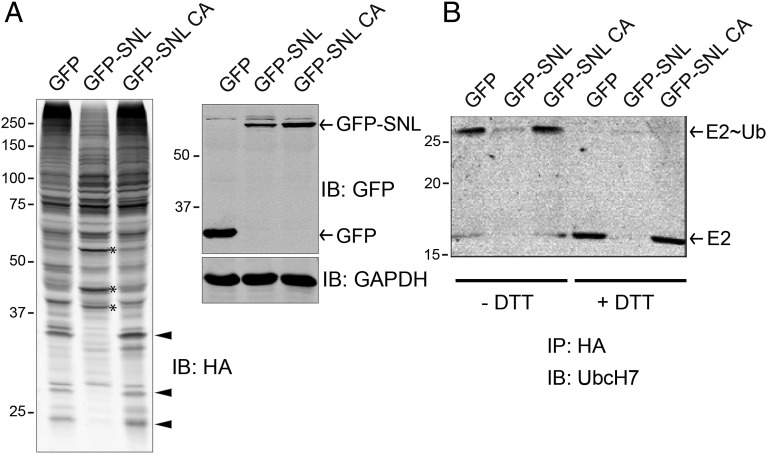
Fig. 2.
Ectopic expression of SidC altered the intracellular ubiquitination pattern. (A) HEK293T cells were cotransfected with HA-ubiquitin and GFP control, GFP-tagged wild-type SidC SNL domain, or its C46A mutant. Whole cell lysates were prepared and analyzed by Western blot with anti-HA (Left), anti-GFP, and anti-GAPDH as a loading control (Right). Arrowheads highlight several bands that are positive in GFP and GFP-SNL C46A controls but are diminished in the presence of wild-type SNL domain. Asterisk denotes bands that are more prominent in the sample expressing wild-type SNL domain. (B) HEK293T cells were cotransfected with HA-ubiquitin and other indicated plasmids. Cells lysates were prepared and incubated with anti-HA beads to immunoprecipitate HA-Ub tagged species. The precipitated samples were prepared in SDS loading buffer without (three lanes on the left) or with (three lanes on the right) DTT and subjected to Western blot analysis. In the presence of DTT, the E2∼Ub complex was fully reduced to E2, indicating a thioester linkage between E2 and ubiquitin.