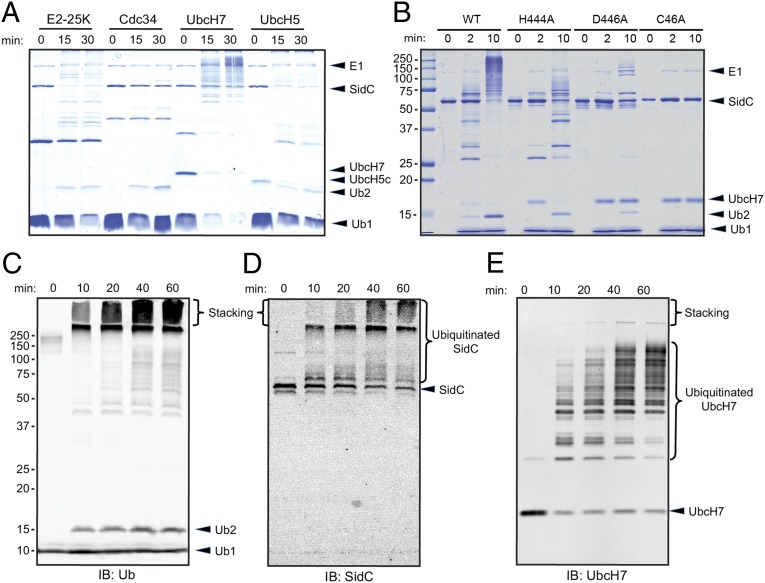
Fig. 3.
The SNL domain of SidC has E3 ubiquitin ligase activity. (A) In vitro ubiquitination assays with the SNL domain of SidC (amino acids 1–542) and 4 representative E2s: E2-25K, Cdc34, UbcH7 and UbcH5. (B) E3 activity assay of SidC H444A, D446A, and C46A mutants. The activity of the C46A mutant was completely abolished, whereas the activities of the H444A and D446A mutants were significantly reduced. (C) Time-dependent ubiquitination assay. The reactions were performed with E1, UbcH7, SNL domain, and wild-type ubiquitin. The reaction mixtures were analyzed by Western blot with anti-ubiquitin antibody. Within the stacking gel, an increased amount of heavily polyubiquitinated species was observed during the time course. (D) Western blot of the same materials as in C with anti-SidC antibody. The high–molecular-weight ubiquitin species were positive for SidC. (E) Western blot of the same samples as in C with anti-UbcH7. UbcH7 was also ubiquitinated during the in vitro reaction.