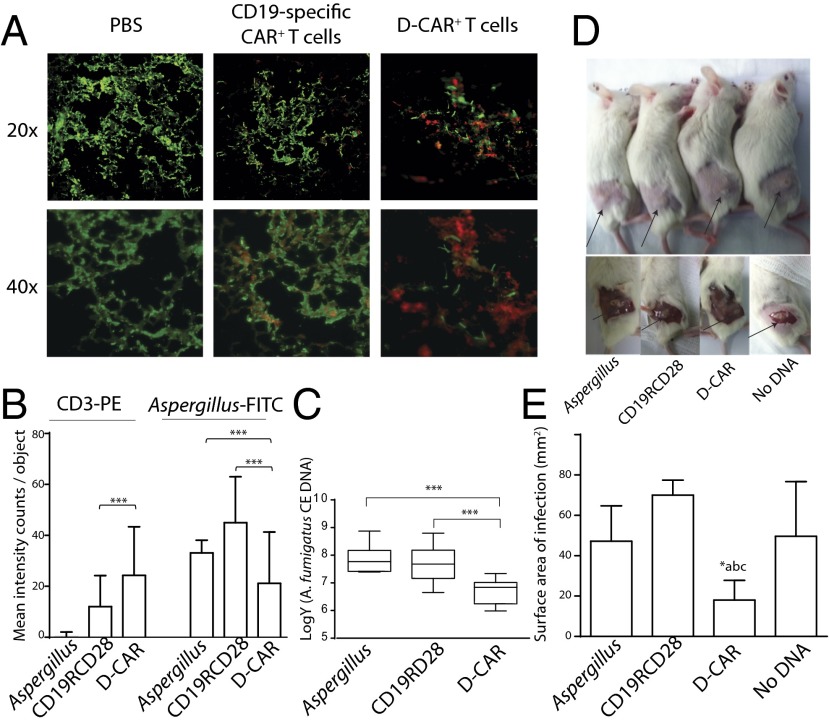
Fig. 4.
Targeting Aspergillus infection in mice by D-CAR+ T cells: (A) Pulmonary Aspergillus infection in NSG mice. Fluorescent microscopic pictures of lung sections of Aspergillus (AF293) in mice that received PBS, CD19-specific CAR+ T cells, and D-CAR+ T cells. (B) Mean fluorescence intensity for staining with anti-human CD3-PE and anti-Aspergillus-FITC was calculated using Inform imaging software. Values are shown as mean fluorescent intensity (counts per image objects) from three lung slides per group. ***P < 0.001. (C) Quantification of A. fumigatus burden in lungs of mice 4 d after infection, as determined by qPCR. Data are expressed as the number of conidial equivalent (CE) copies of DNA in aliquots of lung homogenates. Data are shown as mean ± SD; ***P < 0.001 compared with control mice receiving PBS (labeled “Aspergillus”) or CD19-specific CAR+ T cells; n = 5 mice. (D) Cutaneous Aspergillus infection in NSG mice. Mice received no therapy, CD19-specific (CD19RCD28) CAR+ T cells, D-CAR+ T cells, and T cells expressing no CAR (No DNA). Site of fungal lesions are indicated by arrows. After 96 h, mice were anesthetized, and skin lesions were measured. (E) Cutaneous fungal burden of mice treated with D-CAR+ T cells compared with Aspergillus without treatment, CD19-specific CAR+ T-cells, or (c) no DNA electroporated T-cells. *P < 0.05, treatment with D-CAR+ cells vs. other three conditions; n = 5.