Significance
One of the goals of modern molecular medicine is delivery and expression of heterologous genes in living organisms. RNA-based delivery vectors are a safer choice than DNA vectors, but they are prone to degradation and are highly dependent on efficient delivery methods. One of the ways to improve RNA vector performance is to increase the level of expression of the encoded proteins. We followed this approach and modified standard alphavirus replicon-based expression systems to make the transcribed subgenomic RNA additionally amplifiable by viral replication enzymes. Higher levels of subgenomic RNA synthesis increased the replicons’ expression efficiency at least 10-fold. Such replicons can be widely applied for development of efficient DNA and RNA vaccines and protein production in vitro.
Keywords: expression vectors, vaccines
Abstract
Since the development of infectious cDNA clones of viral RNA genomes and the means of delivery of the in vitro-synthesized RNA into cells, alphaviruses have become an attractive system for expression of heterologous genetic information. Alphaviruses replicate exclusively in the cytoplasm, and their genetic material cannot recombine with cellular DNA. Alphavirus genome-based, self-replicating RNAs (replicons) are widely used vectors for expression of heterologous proteins. Their current design relies on replacement of structural genes, encoded by subgenomic RNAs (SG RNA), with heterologous sequences of interest. The SG RNA is transcribed from a promoter located in the alphavirus-specific RNA replication intermediate and is not further amplified. In this study, we have applied the accumulated knowledge of the mechanism of alphavirus replication and promoter structures, in particular, to increase the expression level of heterologous proteins from Venezuelan equine encephalitis virus (VEEV)-based replicons. During VEEV infection, replication enzymes are produced in excess to RNA replication intermediates, and a large fraction of them are not involved in RNA synthesis. The newly designed constructs encode SG RNAs, which are not only transcribed from the SG promoter, but are additionally amplified by the previously underused VEEV replication enzymes. These replicons produce SG RNAs and encoded proteins of interest 10- to 50-fold more efficiently than those using a traditional design. A modified replicon encoding West Nile virus (WNV) premembrane and envelope proteins efficiently produced subviral particles and, after a single immunization, elicited high titers of neutralizing antibodies, which protected mice from lethal challenge with WNV.
Alphaviruses are a group of enveloped viruses with a positive-strand RNA genome that replicate in most commonly used cell lines to titers exceeding 1010 infectious units (inf.u)/mL (1, 2). Upon infection, the genomic RNA serves as a template for translation of viral nonstructural proteins that form replication complexes (3). Within a few hours postinfection, these complexes synthesize large amounts of viral genomic and subgenomic (SG) RNA (3). The SG RNA is transcribed from the SG promoter and serves as a template for translation of viral structural proteins: capsid, E2 and E1, which ultimately assemble with genomic RNA into infectious viral particles. This highly efficient virus-specific RNA and protein synthesis, coupled with the availability of infectious cDNA clones, have made alphaviruses an attractive system for designing self-replicating vectors for delivery and expression of heterologous genetic information. The most widely used alphavirus-based expression systems are based on replacement of viral structural genes by a gene(s) of interest (4). These modified viral genomes, termed replicons, can be synthesized in vitro and delivered into cells either by transfection or in infectious viral particles, which deliver essentially every packaged RNA molecule into the cells both in vivo and in vitro.
In recent years, significant progress has been made in our understanding of the mechanism of alphavirus replication. Detailed studies have elucidated the structure and function of the RNA promoters, critical aspects of virus–host cell interactions, and the composition of the replication complexes (5–12). These mechanistic studies of alphavirus replication raised the question of whether we are using their entire expression potential, and whether the traditional replicon design can be further improved to achieve higher levels of heterologous protein production. In this project, we sought to apply the latest advances in understanding of alphavirus RNA replication to design a new generation of Venezuelan equine encephalitis virus (VEEV) genome-based expression systems. The distinguishing feature of these constructs is the modification of the SG RNAs. These SG RNAs have been engineered to contain the cis-acting promoter elements, which are normally present at the 5′ end of the viral genome and mediate genomic RNA replication (8, 13, 14). Thus, in these newly designed VEEV replicons (VEErep), the SG RNAs were not only transcribed from the SG promoter, but were capable of replication/amplification by the VEEV replication complexes. As a result, the heterologous gene expression was more efficient than that of the existing constructs, which use replicons with the standard SG RNAs. The expression level of heterologous protein encoded by the improved replicons was also found to be dependent on coexpression of VEEV capsid protein. The VEEV replicons, which use both amplification of the SG RNA and express capsid protein, provide a platform for development of a variety of more efficient expression systems and have numerous applications. To illustrate this, we have generated a VEEV replicon encoding the premembrane and envelope (prM/E) proteins of West Nile virus (WNV). Particles containing the newly designed replicons induced high levels of WNV E protein expression in vitro and elicited robust protective immunity in mice.
Results
Design of More Efficient VEEV-Based Expression System.
Our previous study demonstrated that during VEEV and Sindbis virus infections only a small portion of viral nonstructural proteins (nsPs) is colocalized with dsRNA replication intermediates. Thus, it appears that a large fraction of nsPs are not involved in RNA replication (15–17). This provides an opportunity to exploit the underused ns proteins for amplification of the SG RNAs encoding proteins of interest, which is normally transcribed from the SG promoter and is not further amplified (18).
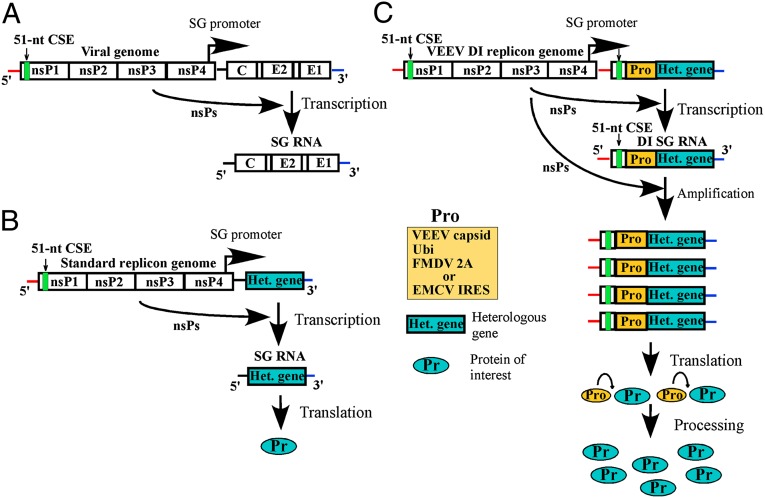
The accumulated experimental evidence has demonstrated that replication/amplification of VEEV and other alphavirus genomes and their defective interfering (DI) RNAs is determined by three promoter elements: (i) the conserved 3′-terminal sequence element (3′ CSE) and the following poly(A) tail; (ii) the 5′ UTR, which functions as a key promoter element for both negative- and positive-strand RNA synthesis; and (iii) the 51-nt conserved sequence element (51-nt CSE), which is located in the nsP1-coding sequence and functions as an enhancer of alphavirus genome replication (5–9). The SG RNA encodes the same 3′ CSE and poly(A) as the viral genome, but contains a different 5′ UTR, and lacks the 51-nt CSE. To test the potentially beneficial effects of the genomic RNA-specific promoter elements on SG RNA synthesis, the 5′ UTR of the VEEV genome and the following fragment, containing the 51-nt CSE, were cloned under control of the SG promoter in the VEEV strain TC-83–based replicon (Fig. 1 and Fig. S1). In this design, we preserved the natural sequence of the SG promoter and the first 2 nt of the subgenomic RNA, but entirely replaced the endogenous SG RNA 5′ UTR with that of the viral genome. Following the new 5′ UTR, we inserted the fragment of the nsP1-coding sequence containing the VEEV-specific 51-nt CSE. The designed nucleotide sequence also had an nsP1-specific initiating AUG (19), and the ORF was fused with the heterologous genes, which we intended to express.
Fig. 1.
The schematic representation of alphavirus vectors and protein expression strategies. (A) The alphavirus genome. (B) Standard alphavirus replicons. Viral structural genes are replaced by heterologous gene of interest. (C) The newly designed VEEV DI replicons. The DI SG RNAs contain all of the promoter elements, which are normally present only in the 5′ end of VEEV genomic RNA (Fig. S1 for details). Presence of these regulatory sequences allows nsPs not only to transcribe the DI SG RNAs but also to amplify these RNA as viral genomes.
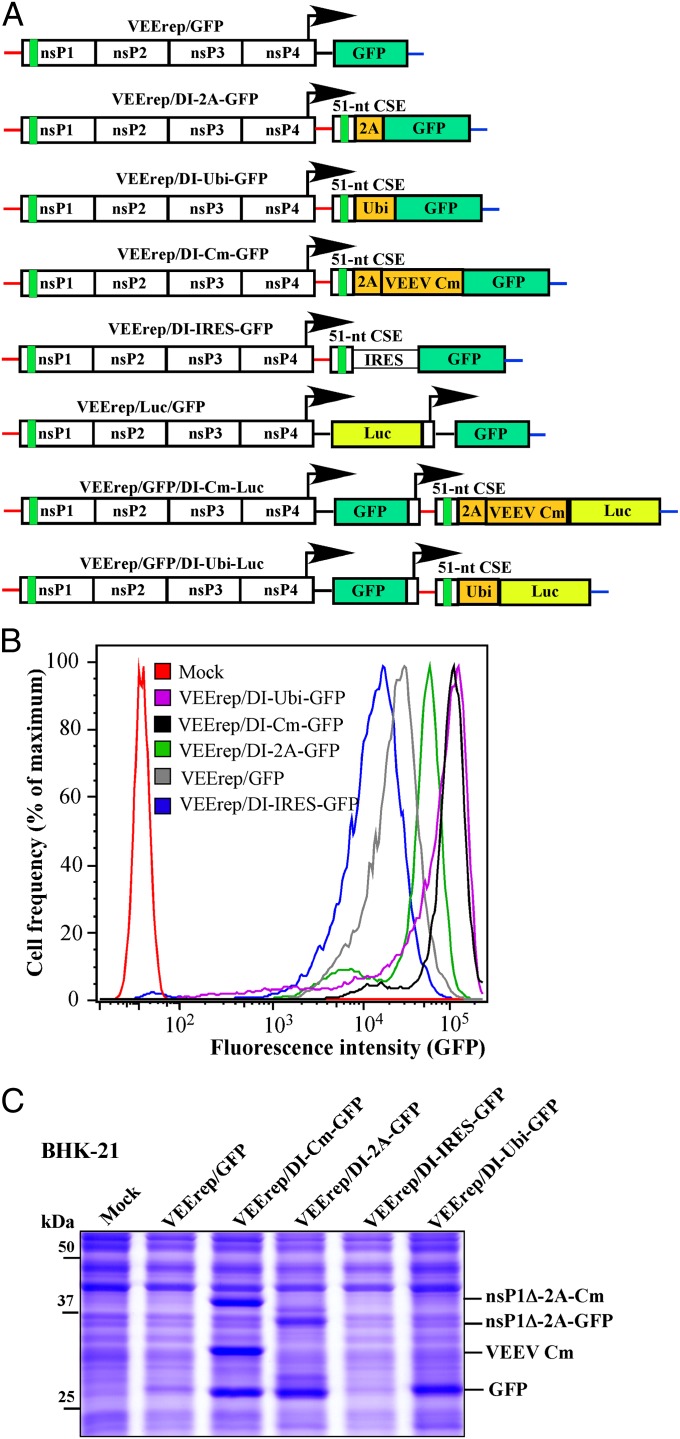
To achieve the expression of the protein of interest without additional amino terminal peptides, the remaining nsP1 fragment and heterologous sequences of interest were separated by genes, whose products are capable of processing the polyprotein in cis. Such genes included ubiquitin (Ubi) (in VEErep/DI-Ubi-GFP), 2A protease of foot-and-mouth-disease virus, FMDV 2A (in VEErep/DI-2A-GFP), and VEEV capsid protein (VEErep/DI-Cm-GFP). The encoded capsid protein sequence used for designing of the latter replicon contained no nuclear localization signal (NLS), which is responsible for its transcription inhibitory functions, and thus, the expressed capsid protein was noncytopathic (10, 19, 20). We also tested whether the encephalomyocarditis virus (EMCV) derived internal ribosome entry site (IRES) could be used for heterologous gene expression. It was used in the VEErep/DI-IRES-GFP construct. To measure changes in heterologous protein expression, in the initial experiments, we used GFP as a protein of interest in our various vector designs. The design of all of the constructs is presented in Fig. 2A. It should be noted that these SG RNAs lacked the packaging signal required for RNA packaging into viral particles. Because the SG RNA strategy resembled that of the previously described DI RNAs (21–23), in this and the following sections, such constructs are referred to as DI replicons. The VEEV TC-83–based VEErep/GFP replicon, having a traditional design (24) and encoding GFP and unmodified SG 5′ UTR under control of the SG promoter, was used as a control to demonstrate a standard level of RNA synthesis and heterologous protein expression.
Fig. 2.
VEEV DI replicons express heterologous proteins more efficiently than those having standard design. (A) The schematic representation of VEEV replicons encoding GFP or Luc genes under control of the subgenomic promoters. (B) BHK-21 cells were infected with the indicated packaged replicons at an MOI of 20 inf.u/cell, and GFP fluorescence was evaluated by FACS analysis at 20 h postinfection. (C) Cells were infected with indicated replicons at the same MOI, harvested at 20 h postinfection, lysed in loading buffer, and samples were analyzed by SDS/PAGE, followed by Coomassie blue staining. Cm, noncytopathic VEEV capsid protein; nsP1Δ, a peptide, whose coding sequence contains a 51-nt CSE.
DI Replicons Demonstrate Higher Levels of Protein Expression.
VEEV replicons used in the study were packaged into viral particles using the previously designed packaging systems (25) and applied for infecting cells at the same multiplicity of infection (MOI). In baby hamster kidney (BHK-21) cells, all of the constructs (Fig. 2A), except that using the EMCV IRES, were more efficient in GFP expression than the standard VEErep/GFP, suggesting amplification of the originally transcribed SG RNA. Unlike VEErep/GFP, they expressed GFP protein to a level readily detectable on Coomassie blue-stained gels as a major protein band, and FACS analysis also demonstrated a strong increase in GFP fluorescence (Fig. 2 B and C). The performance of FMDV-derived 2A protease was not as efficient as we expected, and only ∼50% of GFP was present in a cleaved and free form on the gels (Fig. 2C, see also Fig. 4). The nsP1–capsid and nsP1–GFP fusions were readily detectable on the gels. However, the expressed VEEV capsid protein mediated 100% processing, which did not depend on its presence either in nsP1 fusion or in completely processed form. The lower GFP expression level from the VEErep/DI-IRES-GFP was unexpected, particularly considering the high level of SG RNA synthesis (Fig. 3 for details).
Fig. 4.
DI replicons demonstrate higher levels of heterologous protein synthesis, which correlates with the increase of SG RNA production. Infected cells were metabolically labeled with [35S]methionine at 7 h postinfection (Materials and Methods for details). Fragments of the same gel are presented. Radioactivity in the GFP- and Luc-containing bands was measured on Storm phosphorimager. The data were normalized to the radioactivity in the GFP and Luc bands detected in the VEErep/GFP and VEErep/Luc/GFP samples, respectively.
Fig. 3.
DI replicons demonstrate higher levels of SG RNA synthesis in the infected cells. BHK-21 cells were infected with the indicated packaged replicons at an MOI of 20 inf.u/cell. Replicon-specific RNAs were metabolically labeled with [3H]uridine in the presence of ActD and analyzed by agarose gel electrophoresis (Materials and Methods for details). Panels represent fragments of the same gel and the same X-ray film.
There were noticeable differences in the cytopathogenicity of the designed replicons in all of the cell types that were used. Replicons expressing mutated capsid protein were poorly cytopathic and demonstrated only a cell growth arrest, whereas the VEErep/DI-Ubi-GFP and VEErep/DI-IRES-GFP replicons induced very strong morphological changes of the cells within 12–16 h postinfection. For the VEErep/DI-Ubi-GFP replicon, the abnormally high intracellular concentration of Ubi most likely had a deleterious effect on cell biology.
We also noticed that the level of heterologous protein expression mediated by the designed constructs was specific to the cell type. Most of the constructs produced less GFP in HEK293, Vero, and National Institutes of Health (NIH) 3T3 cells than they did in BHK-21 cells (Fig. S2). However, the VEErep/DI-Cm-GFP was always more productive than a standard VEErep/GFP, suggesting that in the previous studies, the entire potential of VEEV replicons in terms of expression of heterologous proteins was not entirely exploited.
Higher Expression Level of Heterologous Genes Is Not Specific to GFP.
To rule out the possibility that the increased level of protein expression is specific to GFP, we also designed DI replicons expressing a firefly luciferase (Fig. 2A and Fig. S3). The designed replicons demonstrated an even more profound increase in heterologous protein (Luc) expression. At 20 h postinfection, the DI replicons, but not the control VEErep/Luc/GFP, produced quantities of luciferase that were readily detectable on Coomassie blue-stained gels (Fig. S3). Accordingly, at 24 h postinfection, the activity of luciferase in VEErep/GFP/DI-Cm-Luc–infected cells was 50-fold higher than in cells infected with VEErep/GFP/Luc (Fig. S3).
DI Replicons Are Capable of Replication of Subgenomic RNA.
Next we attempted to directly demonstrate that presence of RNA replication promoter elements had a positive impact on subgenomic RNA synthesis. In all cases, the DI RNA-encoding replicons produced their SG RNAs at levels higher than those found in the cells infected with replicons with a standard SG RNA design (Figs. 2A and 3), (see control VEErep/GFP and VEErep/Luc/GFP). The stimulatory effect of the new 5′ promoter sequences on DI SG RNA synthesis was especially evident in the case of Luc-encoding constructs; the DI SG RNAs were synthesized 14- to 24-fold more efficiently than those transcribed from another SG promoter and having natural 5′ UTR. Because both SG RNA promoters were identical, the detected differences in SG RNA synthesis were most likely the result of additional replication of the SG DI RNAs. Moreover, in the cells infected with capsid-expressing replicons VEErep/DI-Cm-GFP and VEErep/DI-Cm-Luc/GFP (Fig. 3), the [3H]-labeled replicon genomic and SG RNAs were found at higher concentrations.
Intracellular Accumulation of Heterologous Proteins Is Not Exclusively Determined by Translation Efficiency.
The final levels of heterologous protein accumulation could be dependent not only on the efficiency of their synthesis, but also on the cytopathic effect (CPE)-associated changes in the intracellular environment, the degradation rates of replicon RNAs, and individual proteins and other parameters. To directly compare the rates of GFP and Luc translation, we metabolically labeled cells infected with different DI replicons with [35S]methionine at 7 h postinfection. The results presented in Fig. 4, demonstrate that GFP and Luc syntheses were particularly efficient in case of Ubi- and FMDV 2A-dependent constructs. At 7 h postinfection, these replicons produced heterologous proteins at rates 30-fold higher than standard VEErep/GFP and VEErep/GFP/Luc (Fig. 4). The Cm-dependent cassettes also synthesized 10- to-20-fold higher levels of the protein of interest. As shown in Fig. 2 and Figs. S2 and S3, the latter constructs were very productive in long-term expression, suggesting that high levels of intracellular protein accumulation were determined both by more efficient synthesis and lower cytopathogenicity of the replicons. Thus, the rate of CPE development appears to be an important parameter, which needs to be considered during optimization of alphavirus replicons for expression of particular proteins.
At 7 h postinfection, all of the used constructs demonstrated different abilities to interfere with cellular translation (Fig. 4). Moreover, expression of capsid protein made replicons incapable of overexpressing VEEV nonstructural proteins nsP1, nsP2, and nsP3. It is likely that in the presence of capsid protein, replicon genomes are efficiently packaged into nucleocapsids, making RNA both resistant to degradation and no longer involved in translation of nsPs. This correlates with the RNA replication data (Fig. 3) and indirectly indicates that the increase in accumulation of VEErep/DI-Cm-GFP and VEErep/DI-Cm-Luc/GFP genomes in the RNA labeling experiments was likely a result of their efficient encapsidation.
DI VEEV Replicons Are More Potent IFN-β Inducers than VEErep/GFP.
Importantly, the designed DI replicons remained potent type I IFN inducers. It has been previously demonstrated that both lack of capsid protein expression in VEEV replicons (26) and inactivation of the capsid-specific NLS in capsid-coding sequence of VEEV (19) made these self-replicating RNAs efficient inducers of antiviral responses. This proinflammatory characteristic allows VEEV replicons to function as potent self-adjuvants. Replication of the subgenomic RNA did not affect replicons’ ability to induce IFN-β. To the contrary, VEErep/DI-Cm-GFP reproducibly induced levels of IFN-β higher than those induced by VEErep/GFP (Fig. S4).
VEEV DI Replicons Can Be Used in Vivo.
It was important to further test whether the newly developed replicons can be applied for in vivo studies. We designed a replicon (Fig. 5A) that encoded WNV prM/E proteins in the context of self-replicating SG DI RNA (VEErep/DI-Cm-WNV). This replicon was packaged into VEEV TC-83–derived structural proteins, and cells infected with this replicon demonstrated accumulation of WNV E protein, some of which was released to the media in form of subviral particles (SVPs) (Fig. 5B), which were readily detected by EM. Intracellular and extracellular E protein was also clearly visible on Coomassie blue-stained gels (Fig. 5C). Efficient accumulation of E protein in the replicon-containing cells was visibly damaging for the endoplasmic reticulum and Golgi networks and was causing profound CPE within 12–16 h postinfection.
Fig. 5.
VEErep/DI-Cm-WNV expresses high level of WNV-specific E protein, which is released from the cells in the SVP form. (A) The schematic representation of VEErep/DI-Cm-WNV replicon, encoding prM and E proteins of WNV. (B) Fractions of the concentrated samples of the media, harvested at 12 h postinfection, were used for negative staining and analyzed by EM. Bar corresponds to 50 nm. (C) SDS/PAGE analysis of WNV E protein present in the cells or released in SVPs at 12 h postinfection. Gel was stained with Coomassie blue.
An initial assessment of the safety and immunogenicity of the VEErep/DI-Cm-WNV replicon was undertaken in 8- to 10-wk-old Swiss Webster mice. Groups of four female mice were inoculated via the i.p. route with a single dose of 108 or 106 inf.u of the packaged VEErep/DI-Cm-WNV replicon, or 104 pfu of an attenuated WNV NY99ic L107F mutant (27). There were no signs of illness in any animals infected at any dose with packaged replicon. Serum samples collected ∼6 wk later showed high titers of neutralizing anti-WNV antibodies in all animals (Table S1). Fifty percent neutralization antibody titers in mice receiving 108 inf.u of packaged VEErep/DI-Cm-WNV ranged between 1,280 and 5,120 and were similar to those in animals receiving the attenuated live virus, whereas titers for the 106 group were detectably lower.
Subsequently, groups of five or eight 3- to 4-wk-old mice were inoculated i.p. with single doses of VEErep/DI-Cm-WNV ranging between 108 and 102 inf.u, or with PBS only. All mice were bled at 19 d postimmunization and challenged at 22 d with a lethal dose of WNV strain NY99 (100 pfu, which is equivalent to ∼100 LD50). Dose-dependent differences in neutralizing antibody titers and survival rates were observed, with 100% seroconversion and survival in the 108 and 106 groups (Fig. 6). Neutralizing antibody titers were lower in those groups than had been observed in the initial experiment, which could likely be explained by the shorter interval postimmunization for sample collection.
Fig. 6.
Neutralizing antibody titers (50% focus reduction) and protection among 3- to 4-wk-old Swiss Webster mice inoculated with a single dose of VEErep/DI-Cm-WNV and challenged with 100 LD50 of WNV NY99. Animals were bled at 19 d postimmunization and challenged at 22 d postimmunization. n/a, not applicable.
Progressively fewer seroconvertors/survivors were seen at the two lower doses of VEErep/DI-Cm-WNV replicon. However, despite only three of eight mice in the 104-dose group having detectable prechallenge neutralizing antibodies, an 85% survival rate was observed. All but one of the animals that survived challenge showed a more than twofold increase in antibody titer in the postchallenge sample, although increases in titer were slightly lower in the 108-dose group compared with the lower dose groups.
Discussion
In recent years, the accumulated knowledge of the molecular mechanisms of alphavirus replication and virus–host interactions has reached a level, which facilitates targeted manipulations of their genomes to achieve a programmed phenotype (28). Besides causing infections of significant public health threat (29, 30), alphaviruses have been previously modified into RNA vector systems (4), which can deliver and express additional genetic information both in vivo and in vitro. To date, strategies used for alphavirus vector development have been very straightforward. Their structural genes in the SG RNA were either replaced by genes of interest or the subgenomic promoters were duplicated to control the expression of additional genes (4). Similarly to alphavirus genomes, replicon RNAs, which encode no structural genes, replicate exclusively in the cytoplasm and cannot introduce their genetic material into the cellular genome. They can be used either for transient expression of proteins of interest or for producing stable cell lines expressing heterologous proteins from persistently replicating RNAs (24, 31). However, the most important use of replicons appears to be their application for development of recombinant vaccines (32–36). Among the alphaviruses, VEEV replicons became more widely used for these applications because of their higher levels of protein expression, their abilities to serve as self-adjuvants (37, 38), and because they efficiently replicate in human cells.
Our data suggest that replacement of the structural genes in the subgenomic RNA is not the end point of VEEV genome-based vector development. They can be additionally modified to achieve higher levels of protein expression. The coding strategy of VEEV replicon genomes can be changed in such a way that the transcribed SG RNAs function as templates for replicon-encoded viral replication complexes. Thus, the number of intracellular SG RNAs can be significantly increased and, consequently, the encoded proteins are synthesized more efficiently. This approach was found to be successful and the newly designed DI replicons were capable of synthesizing proteins of interest to 10- to 20-fold higher levels than standard VEEV replicons. Importantly, they remain efficient inducers of type I IFN and, probably, the proinflammatory cytokines. Single immunization of mice with replicons expressing prM/E proteins of WNV was sufficient to induce readily detectable levels of neutralizing antibodies and protect mice against subsequent infection with highly pathogenic WNV. VEErep/DI-Cm-WNV was able not only to express high levels of intracellular WNV-specific proteins, but also to produce SVPs, which presumably contributed to the robust immunogenicity of this replicon. Taken together, the data demonstrated that these replicons retained all of the advantages of previously designed VEEV-based expression systems, but also became more productive in terms of heterologous protein synthesis.
Despite these significant improvements, there appears to be possibilities for further increase of the efficacy of these new VEEV-based replicons. Unexpectedly, the VEEV capsid protein was found to be applicable not only as a self-protease, but also as an enhancer of replicon genome and SG RNA synthesis. First, it is possible that similarly to the capsid protein of rubella virus (39), it may function as a component of the replication complex. Second, it may package viral genomes and subgenomic RNAs into nucleocapsids and thus increase their stability. The SG RNAs contain no packaging signal (40). Nevertheless, the possibility of their partial encapsidation in the presence of capsid protein cannot be ruled out. If this is the case, higher levels of protein expression from the designed replicon also raises the question as to whether RNA packaging into nucleocapsids is a reversible process and whether there is a dynamic balance between RNA packaging and release.
In summary, this study demonstrated that VEEV-based vectors are flexible in terms of introducing additional modifications that increase the expression levels of the encoded proteins. Depending on the cell line used for expression, it is possible to use different genes in the constructs, whose products mediate processing of the initially translated fusion protein and to modify the replicon’s ability to induce CPE. We have tested only a limited number of such genes. However, the modified, noncytopathic VEEV capsid protein used in this study appears to be reasonably universal in promoting efficient cleavage in multiple cell lines and additional up-regulation of RNA replication. The enhanced synthesis of SG RNAs and the encoded protein can be used to increase the efficiency of VEEV replicon-based DNA vaccines, which are currently in development, and RNA vaccines in particular. The low stability of RNA vaccines during their delivery in vivo can be at least partially compensated by achieving higher levels of heterologous protein expression.
Materials and Methods
Plasmid Constructs.
The DI SG RNAs encoded 228 nt of the 5′ terminus of VEEV TC-83 genome followed by sequences of different proteases and heterologous genes of interest. All of the constructs were designed using standard PCR-mediated techniques. To increase translation, all of the AUG codons in the nsP1 sequence cassette were placed in-frame with the capsid gene by deleting nucleotides 129 and 191 (19). WNV cassette encoding signal peptide, prM and E sequences of WNV NY99, was derived from the plasmid described elsewhere (41). Standard recombinant DNA techniques were applied for the construction of the plasmids. pVEErep/GFP and pVEErep/Luc/GFP plasmids were designed based on VEEV TC-83 replicons and have been described elsewhere (13, 24). Helper genome constructs encoding VEEV TC-83 capsid protein and glycoproteins have been described elsewhere (25).
Analysis of Protein and RNA Synthesis.
The 5 × 105 (BHK-21, NIH 3T3, Vero, or HEK293) cells in six-well Costar plates were infected with packaged replicon genomes at an MOI of 20 inf.u/mL. For analysis of long-term protein expression, cells were harvested at 20 h postinfection and lysed in protein loading buffer. Equal amounts of samples were analyzed by SDS/PAGE and then stained with Coomassie blue. For FACS analysis, cells were trypsinized at 20 h postinfection, fixed with 4% paraformaldehyde, and fluorescence was measured by flow cytometry using LSR II (BD Biosciences). For analysis of the rates of protein synthesis, BHK-21 cells were metabolically labeled with [35S]methionine at 7 h postinfection for 30 min in 0.8 mL of DMEM lacking methionine, supplemented with 0.1% FBS and 20 μCi/mL of [35S]methionine. Equal amounts of proteins were loaded onto SDS/PAGE. After electrophoresis, gels were dried and autoradiographed. Quantitative analysis of radioactivity in the specific bands was performed using a Storm phosphorimager. To analyze synthesis of replicon-specific RNAs, at 3 h postinfection, media in the wells were replaced by 0.8 mL of αMEM supplemented with 10% FBS, actinomycin D (1 μg/mL), and [3H]uridine (20 μCi/mL). After 4 h of incubation at 37 °C, total cellular RNAs were isolated with TRizol (Invitrogen) according to the manufacturer’s protocol, then denatured with glyoxal in dimethyl sulfoxide, and analyzed by agarose gel electrophoresis using the previously described conditions (17). Gels were impregnated with 2,5-diphenyloxazole (PPO), dried, and autoradiographed.
WNV SVP Analysis.
BHK-21 cells were infected with packaged VEErep/DI-Cm-WNV replicon at an MOI of 20 inf.u/cell. At 6 h postinfection cells were washed and overlaid with serum-free medium (VP-SFM; Gibco). Media was harvested at 12 h postinfection, and particles were concentrated using centrifugal Ultracel-100K filters (Millipore). Then they were either pelleted by ultracentrifugation at 50,000 rpm for 1 h at 4 °C in a TLA-55 rotor using a TL-100 tabletop ultracentrifuge (Beckman) for further analysis by 10% SDS/PAGE or used for EM analysis (42). Droplets of virus suspension were placed onto copper electron microscope grids and stained with 1% uranyl acetate. Images were acquired at magnification of 50,000× using a FEI Tecnai F20 electron microscope in the University of Alabama at Birmingham’s cryo-EM core facility.
Supplementary Material
Acknowledgments
The authors thank Maricela Ramos for technical assistance and Dr. Niall Foy for critical reading of the manuscript. Studies at the University of Alabama at Birmingham were supported by National Institutes of Health (NIH) Grants R01AI070207, R01AI095449, and R01AI073301. Studies at the University of Texas Medical Branch (UTMB) were supported by funding from the Institute for Human Infections and Immunity (to D.W.C.B.). A.J.M. was supported by a predoctoral fellowship from the Jeane B. Kempner scholar program at UTMB. J.A.P. was supported by a predoctoral fellowship from the Biodefense Training Program, NIH Grant T32-AI060549.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1408677111/-/DCSupplemental.
References
- 1.Griffin DE. Alphaviruses. In: Knipe DM, Howley PM, editors. Fields' Virology. 4th Ed. New York: Lippincott, Williams and Wilkins; 2001. pp. 917–962. [Google Scholar]
- 2.Weaver SC, Frolov I. Togaviruses. In: Mahy BWJ, Meulen VT, editors. Virology. Vol 2. Salisbury, UK: ASM Press; 2005. pp. 1010–1024. [Google Scholar]
- 3.Strauss EG, Strauss JH. Structure and replication of the alphavirus genome. In: Schlesinger S, Schlesinger MJ, editors. The Togaviridae and Flaviviridae, The Viruses. New York: Plenum; 1986. pp. 35–90. [Google Scholar]
- 4.Frolov I, et al. Alphavirus-based expression vectors: Strategies and applications. Proc Natl Acad Sci USA. 1996;93(21):11371–11377. doi: 10.1073/pnas.93.21.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frolov I, Hardy R, Rice CM. Cis-acting RNA elements at the 5′ end of Sindbis virus genome RNA regulate minus- and plus-strand RNA synthesis. RNA. 2001;7(11):1638–1651. doi: 10.1017/s135583820101010x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fayzulin R, Frolov I. Changes of the secondary structure of the 5′ end of the Sindbis virus genome inhibit virus growth in mosquito cells and lead to accumulation of adaptive mutations. J Virol. 2004;78(10):4953–4964. doi: 10.1128/JVI.78.10.4953-4964.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorchakov R, Hardy R, Rice CM, Frolov I. Selection of functional 5′ cis-acting elements promoting efficient sindbis virus genome replication. J Virol. 2004;78(1):61–75. doi: 10.1128/JVI.78.1.61-75.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michel G, Petrakova O, Atasheva S, Frolov I. Adaptation of Venezuelan equine encephalitis virus lacking 51-nt conserved sequence element to replication in mammalian and mosquito cells. Virology. 2007;362(2):475–487. doi: 10.1016/j.virol.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strauss JH, Kuhn RJ, Niesters HGM, Strauss EG. Functions of the 5′-terminal and 3′-terminal sequences of the Sindbis virus genome in replication. In: Brinton MA, Heinz FX, editors. New Aspects of Positive-Strand RNA Viruses. Washington, DC: American Society of Microbiology; 1990. pp. 61–66. [Google Scholar]
- 10.Atasheva S, Fish A, Fornerod M, Frolova EI. Venezuelan equine encephalitis virus capsid protein forms a tetrameric complex with CRM1 and importin alpha/beta that obstructs nuclear pore complex function. J Virol. 2010;84(9):4158–4171. doi: 10.1128/JVI.02554-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garmashova N, et al. Analysis of Venezuelan equine encephalitis virus capsid protein function in the inhibition of cellular transcription. J Virol. 2007;81(24):13552–13565. doi: 10.1128/JVI.01576-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garmashova N, et al. The Old World and New World alphaviruses use different virus-specific proteins for induction of transcriptional shutoff. J Virol. 2007;81(5):2472–2484. doi: 10.1128/JVI.02073-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kulasegaran-Shylini R, Atasheva S, Gorenstein DG, Frolov I. Structural and functional elements of the promoter encoded by the 5′ untranslated region of the Venezuelan equine encephalitis virus genome. J Virol. 2009;83(17):8327–8339. doi: 10.1128/JVI.00586-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulasegaran-Shylini R, Thiviyanathan V, Gorenstein DG, Frolov I. The 5’UTR-specific mutation in VEEV TC-83 genome has a strong effect on RNA replication and subgenomic RNA synthesis, but not on translation of the encoded proteins. Virology. 2009;387(1):211–221. doi: 10.1016/j.virol.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorchakov R, Garmashova N, Frolova E, Frolov I. Different types of nsP3-containing protein complexes in Sindbis virus-infected cells. J Virol. 2008;82(20):10088–10101. doi: 10.1128/JVI.01011-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frolova EI, Gorchakov R, Pereboeva L, Atasheva S, Frolov I. Functional Sindbis virus replicative complexes are formed at the plasma membrane. J Virol. 2010;84(22):11679–11695. doi: 10.1128/JVI.01441-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorchakov R, et al. A new role for ns polyprotein cleavage in Sindbis virus replication. J Virol. 2008;82(13):6218–6231. doi: 10.1128/JVI.02624-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strauss JH, Strauss EG. The alphaviruses: Gene expression, replication, and evolution. Microbiol Rev. 1994;58(3):491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atasheva S, et al. Pseudoinfectious Venezuelan equine encephalitis virus: A new means of alphavirus attenuation. J Virol. 2013;87(4):2023–2035. doi: 10.1128/JVI.02881-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atasheva S, Garmashova N, Frolov I, Frolova E. Venezuelan equine encephalitis virus capsid protein inhibits nuclear import in mammalian but not in mosquito cells. J Virol. 2008;82(8):4028–4041. doi: 10.1128/JVI.02330-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levis R, Weiss BG, Tsiang M, Huang H, Schlesinger S. Deletion mapping of Sindbis virus DI RNAs derived from cDNAs defines the sequences essential for replication and packaging. Cell. 1986;44(1):137–145. doi: 10.1016/0092-8674(86)90492-7. [DOI] [PubMed] [Google Scholar]
- 22.Lehtovaara P, Söderlund H, Keränen S, Pettersson RF, Kääriäinen L. 18S defective interfering RNA of Semliki Forest virus contains a triplicated linear repeat. Proc Natl Acad Sci USA. 1981;78(9):5353–5357. doi: 10.1073/pnas.78.9.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehtovaara P, Söderlund H, Keränen S, Pettersson RF, Kääriäinen L. Extreme ends of the genome are conserved and rearranged in the defective interfering RNAs of Semliki Forest virus. J Mol Biol. 1982;156(4):731–748. doi: 10.1016/0022-2836(82)90139-5. [DOI] [PubMed] [Google Scholar]
- 24.Petrakova O, et al. Noncytopathic replication of Venezuelan equine encephalitis virus and eastern equine encephalitis virus replicons in Mammalian cells. J Virol. 2005;79(12):7597–7608. doi: 10.1128/JVI.79.12.7597-7608.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volkova E, Gorchakov R, Frolov I. The efficient packaging of Venezuelan equine encephalitis virus-specific RNAs into viral particles is determined by nsP1-3 synthesis. Virology. 2006;344(2):315–327. doi: 10.1016/j.virol.2005.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konopka JL, Thompson JM, Whitmore AC, Webb DL, Johnston RE. Acute infection with venezuelan equine encephalitis virus replicon particles catalyzes a systemic antiviral state and protects from lethal virus challenge. J Virol. 2009;83(23):12432–12442. doi: 10.1128/JVI.00564-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang S, et al. A mutation in the envelope protein fusion loop attenuates mouse neuroinvasiveness of the NY99 strain of West Nile virus. Virology. 2006;353(1):35–40. doi: 10.1016/j.virol.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 28.Kim DY, et al. Design of chimeric alphaviruses with a programmed, attenuated, cell type-restricted phenotype. J Virol. 2011;85(9):4363–4376. doi: 10.1128/JVI.00065-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffin DE. Alphavirus pathogenesis and immunity. In: Schlesinger S, Schlesinger MJ, editors. The Togaviridae and Flaviviridae, The Viruses. New York: Plenum; 1986. pp. 209–250. [Google Scholar]
- 30.Weaver SC, Tesh RB, Shope RE. Alphaviruses (VEE) In: Guerrant RL, Krogstad DJ, Maguire JH, Walker DH, Weller PF, editors. Tropical Infectious Diseases: Principles, Pathogens and Practice. Vol 2. New York: Churchill Livingstone; 1998. pp. 1281–1287. [Google Scholar]
- 31.Frolov I, et al. Selection of RNA replicons capable of persistent noncytopathic replication in mammalian cells. J Virol. 1999;73(5):3854–3865. doi: 10.1128/jvi.73.5.3854-3865.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baric RS, et al. Expression and self-assembly of Norwalk virus capsid protein from Venezuelan equine encephalitis virus replicons. J Virol. 2002;76(6):3023–3030. doi: 10.1128/JVI.76.6.3023-3030.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnston RE, et al. Vaccination of macaques with SIV immunogens delivered by Venezuelan equine encephalitis virus replicon particle vectors followed by a mucosal challenge with SIVsmE660. Vaccine. 2005;23(42):4969–4979. doi: 10.1016/j.vaccine.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 34.Mok H, et al. Venezuelan equine encephalitis virus replicon particles encoding respiratory syncytial virus surface glycoproteins induce protective mucosal responses in mice and cotton rats. J Virol. 2007;81(24):13710–13722. doi: 10.1128/JVI.01351-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas CE, et al. Vaccination of mice with gonococcal TbpB expressed in vivo from Venezuelan equine encephalitis viral replicon particles. Infect Immun. 2006;74(3):1612–1620. doi: 10.1128/IAI.74.3.1612-1620.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu W, et al. Comparison of immune responses to gonococcal PorB delivered as outer membrane vesicles, recombinant protein, or Venezuelan equine encephalitis virus replicon particles. Infect Immun. 2005;73(11):7558–7568. doi: 10.1128/IAI.73.11.7558-7568.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson JM, et al. Mucosal and systemic adjuvant activity of alphavirus replicon particles. Proc Natl Acad Sci USA. 2006;103(10):3722–3727. doi: 10.1073/pnas.0600287103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson JM, Whitmore AC, Staats HF, Johnston RE. Alphavirus replicon particles acting as adjuvants promote CD8+ T cell responses to co-delivered antigen. Vaccine. 2008;26(33):4267–4275. doi: 10.1016/j.vaccine.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tzeng WP, Matthews JD, Frey TK. Analysis of rubella virus capsid protein-mediated enhancement of replicon replication and mutant rescue. J Virol. 2006;80(8):3966–3974. doi: 10.1128/JVI.80.8.3966-3974.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim DY, Firth AE, Atasheva S, Frolova EI, Frolov I. Conservation of a packaging signal and the viral genome RNA packaging mechanism in alphavirus evolution. J Virol. 2011;85(16):8022–8036. doi: 10.1128/JVI.00644-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mason PW, Shustov AV, Frolov I. Production and characterization of vaccines based on flaviviruses defective in replication. Virology. 2006;351(2):432–443. doi: 10.1016/j.virol.2006.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lulla V, Kim DY, Frolova EI, Frolov I. The amino-terminal domain of alphavirus capsid protein is dispensable for viral particle assembly but regulates RNA encapsidation through cooperative functions of its subdomains. J Virol. 2013;87(22):12003–12019. doi: 10.1128/JVI.01960-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.