Abstract
Objective
Elevated plasma homocysteine (tHcy) and the MTHFR c.677C > T variant have been postulated to increase the risk of venous thromboembolism (VTE), although mechanisms and implications to pediatrics remain incompletely understood. The objectives of this study were to determine the prevalences of elevated tHcy and MTHFR variant in a pediatric population with VTE or arterial ischemic stroke (AIS), and to determine associations with thrombus outcomes.
Study Design
Subjects were enrolled in an institution-based prospective cohort of children with VTE or AIS. Inclusion criteria consisted of objectively confirmed thrombus, ≤21 years at diagnosis, tHcy measured and MTHFR c.677C > T mutation analysis. Clinical and laboratory data were collected. Frequencies for elevated tHcy and MTHFR variant were compared with NHANES values for healthy US children and also between study groups (VTE vs AIS, provoked vs idiopathic) and by age.
Results
The prevalences of hyperhomocysteinemia or MTHFR variant were not increased in comparison to NHANES. tHcy did not differ between those with wild-type MTHFR versus either c.677C > T heterozygotes or homozygotes. There was no association between tHcy or MTHFR variant and thrombus outcomes.
Conclusion
In this cohort of US children with VTE or AIS, neither the prevalence of hyperhomocysteinemia nor that of MTHFR variant was increased relative to reference values, and adverse thrombus outcomes were not definitively associated with either. While it is important to consider that milder forms of pyridoxine-responsive classical homocystinuria will be detected only by tHcy, we suggest that routine testing of MTHFR c.677C > T genotype as part of a thrombophilia evaluation in children with incident thromboembolismis not warranted until larger studies have been performed in order to establish or refute a link between MTHFR and adverse outcomes.
Keywords: MTHFR c.677C >T, Hyperhomocysteinemia, Venous thromboembolism, Arterial ischemic stroke, Children, Thrombophilia
Introduction
The role of hyperhomocysteinemia in vascular and thromboembolic disease has been researched and widely debated since 1969 when McCully described significant vascular disease in patients with markedly elevated plasma homocysteine levels (tHcy) [1]. tHcy is thought to increase thrombotic risk by inducing endothelial injury in venous and arterial vasculature; however, the precise prothrombotic mechanisms are poorly understood [2].While highly elevated tHcy, as seen in rare disorders such as cystathionine beta-synthase deficiency, has clearly been shown to cause both arterial and venous thrombosis in up to 25% of untreated patients, evidence regarding the risk of mild elevations of homocysteine is less clear [3,4]. For example, a pilot study showed that supplemental folate reduced tHcy; a subsequent randomized, double-blind, placebo-controlled trial of B-vitamin supplementation, while successful in lowering tHcy, did not decrease the risk of venous thromboembolism (VTE) in adults [5,6].
The thermolabile variant of the methylenetetrahydrofolate reductase (MTHFR c.677C > T) gene has been shown to have decreased enzyme activity in vitro, and has been established as a common genetic cause of mild hyperhomocysteinemia in European epidemiological studies [7]. Such studies have also implicated MTHFR c.677C > T as a risk factor for incident VTE in adults. However, these findings have not been clearly substantiated in studies of populations, particularly in the U.S., where flour products are supplemented with folate [8]. Several recent studies have suggested that MTHFR c.677C > T is common and not a significant risk factor for VTE [8,9]. Nevertheless, testing for MTHFR c.677C > T and tHcy has become widespread in U.S. patients with VTE.
The objectives of this study were to evaluate in a U.S. pediatric population with thromboembolic disease: (1) the prevalence of elevated tHcy, and MTHFR c.677C > T heterozygosity and homozygosity in comparison with published age-appropriate controls; (2) the distribution of tHcy in MTHFR c.677C > T polymorphisms versus wild-type; and (3) an association between tHcy or MTHFR c.677C > T with adverse thrombus outcomes (progression/recurrence or post-thrombotic syndrome [PTS]).
Methods
Subjects
After providing signed informed consent/assent, subjects were enrolled and followed in an institution-based prospective cohort study of pediatric VTE and arterial ischemic stroke (AIS) between April 2006 and June 2010, inclusive (Colorado Multiple Institutional Review Board #05-0339). Eligibility criteria for the present analysis were: 1) VTE or AIS confirmed by objective imaging; 2) age ≤ 21 years at diagnosis; 3) tHcy measured 30 days prior to, or any time after, VTE/AIS diagnosis; and 4) MTHFR c.677C > T mutation analysis. While tHcy testing was performed based on clinical indications and not by study protocol, an electronic medical record (EMR)-based order set for diagnostic thrombophilia testing, established in accordance with the local standard of care, called for tHcy testing for all newly-diagnosed VTE and AIS patients (i.e., at the time of acute hospitalization or at the 1 month follow-up visit). Children were on a usual US diet which includes B12 fortification of breakfast cereals and folate supplementation of all enriched breads, cereals, flours, corn meals, pastas and rice.
Data Extraction
For all patients who met the first three eligibility criteria, tHcy levels were extracted. The National Health and Nutrition Examination Survey (NHANES) data for tHcy plasma levels in 3992 healthy US children were used as control age-matched reference values [10]. For those patients who met all four criteria, additional clinical data were extracted, including demographics, thrombus incident type (VTE or AIS), vascular distribution (for VTE events), known clinical risk factors for VTE or AIS, recurrent/progressive VTE or recurrent AIS, and development of PTS for VTE events affecting the venous drainage of a limb. VTE progression was defined by either contiguous extension of thrombus or evolution to complete occlusion from a previously non-occlusive thrombus within one month of initial VTE diagnosis. Recurrent thromboembolism was defined as a new thromboembolism not meeting the definition of progression, and included recurrent AIS. PTS was evaluated in children with VTE using a standardized, validated pediatric outcome instrument as previously described [11,12]. PTS was categorized as “any PTS” if the subject had an abnormal pain score or findings on physical exam (venous collaterals, edema, etc.). PTS was categorized as “clinically significant PTS,” only if both pain and physical findings were present, as previously reported [11,13].
Laboratory Testing
Blood samples were collected in a non-fasting state. All samples were immediately transported to the clinical laboratory at room temperature via tube system, centrifuged, aliquoted and frozen within one hour of draw. tHcy plasma concentration was assayed using capillary gas chromatography–mass spectrometry or with an enzymatic reaction using the Vitros 5600 analyzer; both of these methods are highly sensitive to mild elevations in tHcy [14,15]. MTHFR c.677C > T, was determined using polymerase chain reaction [PCR]. In accordance with the local standard of care, the electronic medical record (EMR)-based diagnostic thrombophilia testing order sets also called for measurement of antithrombin, protein C, free protein S, factor V Leiden, prothrombin c. 20210G > A polymorphism, factor VIII activity, the lupus anticoagulant, and anticardiolipin and anti-beta-2-glycoprotein-I IgG and IgM antibodies. Data on the MTHFR c.1298A > C variant, if performed, were also extracted.
Statistical Analysis
Statistical analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC, USA). Continuous data were reported as medians with ranges, and dichotomous data as frequencies (proportions, percents). Data distributions were compared between groups by Mann–Whitney U test, and frequencies were compared by chi-squared or Fisher’s exact test, as appropriate. For all inferential statistics, a two-tailed P-value less than 0.05 was considered statistically significant. Odds ratios were generated for the differences in adverse outcomes between groups, in order to estimate sample sizes needed for power analyses.
Results
Study Population
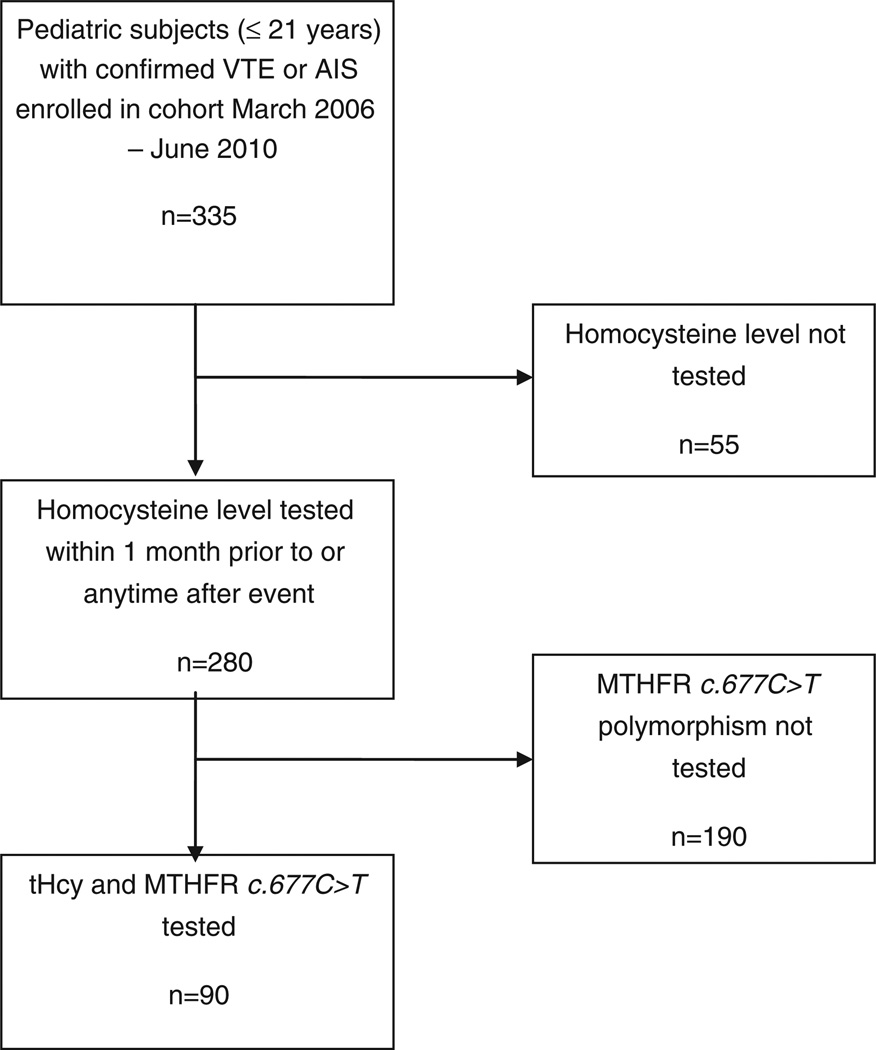
Fig. 1 provides a flow diagram of patient selection according to study eligibility criteria. A total of 335 children with confirmed VTE or AIS were enrolled in the prospective cohort during the time period of this study. Of these, 55 were excluded due to lack of tHcy testing, testing by a less sensitive method or testing not in the specified time period. For the remaining 280 children, tHcy levels were extracted, and it was determined if MTHFR c.677C > T genetic testing was done. A total of 90 children met full study eligibility criteria, and further clinical and laboratory data were collected and analyzed for these 90 children. Characteristics of these 90 study children are displayed in Table 1. Median age for the VTE group was 15.5 years (range: birth-21 years) and for the AIS group was 0.6 years (range: birth-18 years). Overall, 62% of the population had at least one identified clinical prothrombotic risk factor.
Fig. 1.
Flow chart demonstrating selection of study group according to inclusion and exclusion criteria. From an initial 335 children with thromboembolism enrolled into the cohort over 51 months, 90 had both evaluable tHcy and MTHFR c.677C > T genotype determined, and thus were included in the final analysis.
Table 1. Patient and event characteristics in the study population.
Data are given as counts, followed by percents in parentheses, except as otherwise noted.
| Arterial ischemic stroke (AIS) n=47 |
Venous thromboembolism (VTE) n=43 |
Overall (n = 90) | |
|---|---|---|---|
| Gender (female) | 16 (34%) | 22 (51%) | 38 (42%) |
| Race (self reported) | |||
| Caucasian (includes Hispanic) | 44 (94%) | 36 (84%) | 80 (89%) |
| Black | 0 | 6 (14%) | 6 (7%) |
| Native American/Native Alaskan | 2 (4%) | 0 | 2 (2%) |
| Other/more than one race | 1 (2%) | 1 (2%) | 2 (2%) |
| Asian | 0 | 0 | 0 |
| Native Hawaiian/Pacific Islander | 0 | 0 | 0 |
| Kidney disease | |||
| Acute | 1 (2%) | 2 (5%) | 3 (3%) |
| Chronic | 1 (2%) | 0 | 2 (2%) |
| Overt clinical prothrombotic risk factors1 | |||
| None | 28 (60%) | 6 (14%) | 34 (38%) |
| Confirmed infection (including cystic fibrosis) | 6 (13%) | 15 (35%) | 21 (23%) |
| Hospitalization/prolonged immobility | 1 (2%) | 12 (25%) | 13 (14%) |
| Catheter at site of thrombus | 1 (2%) | 11 (26%) | 12 (13%) |
| Vascular abnormality (Moyamoya, May Thurner, vasculitis) | 8 (17%) | 2 (5%) | 10 (11%) |
| Cardiac condition (congenital or acquired) | 7 (15%) | 2 (5%) | 9 (10%) |
| Trauma | 4 (9%) | 4 (9%) | 8 (9%) |
| Chronic inflammatory conditions (SLE, IBD) | 1 (2%) | 7 (16%) | 8 (9%) |
| Dehydration | 2 (4%) | 4 (9%) | 6 (7%) |
| Surgery | 2 (4%) | 3 (7%) | 5 (6%) |
| Oral contraceptive/estrogen use | 0 | 5 (12%) | 5 (6%) |
| Pregnancy | 0 | 4 (9%) | 4 (4%) |
| Malignancy | 1 (2%) | 2 (5%) | 3 (3%) |
| Acidosis | 0 | 2 (5%) | 2 (2%) |
| Smoking | 0 | 1 (2%) | 1 (1%) |
| Travel | 0 | 0 | 0 |
| Other Genetic Thrombophilias2 | |||
| Factor V Leiden | |||
| Heterozygous | 4 (9%) | 7 (16%) | 11 (12%) |
| Homozygous | 0 | 0 | 0 |
| Prothrombin G20210A | |||
| Heterozygous | 3 (6%) | 2 (5%) | 5 (6%) |
| Homozygous | 0 | 0 | 0 |
| Antithrombin deficiency | 0 | 0 | 0 |
| Protein C deficiency | 0 | 0 | 0 |
| Protein S deficiency | 0 | 3 (7%) | 3 (3%) |
Percentage totals may exceed 100% due to presence of more than one risk factor in many patients.
Two subjects had 2 thrombophilias each.
Extremity clots only, 20 subjects assessed.
Homocysteine Levels
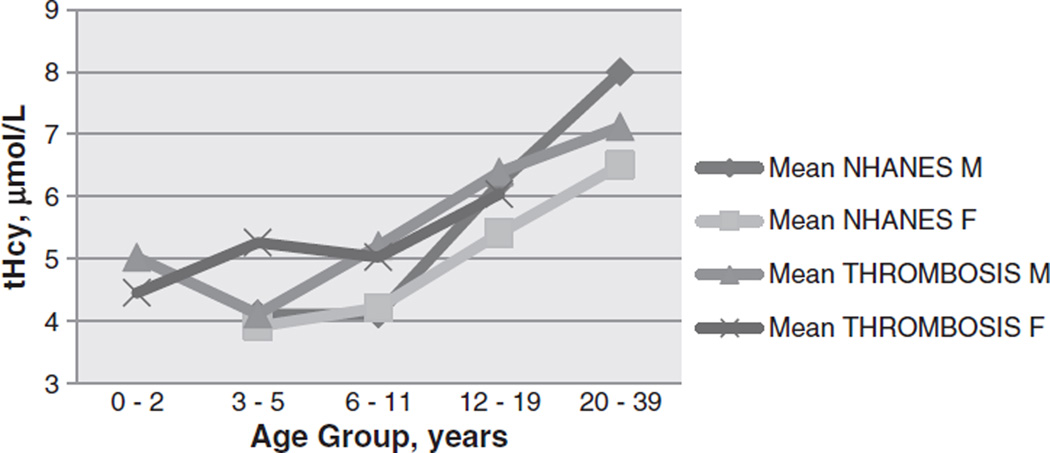
The overall median plasma tHcy for the 280 children with VTE or AIS was 5.35 µmol/L (range: <2 – 16). Six (2.1%) of the 280 children had tHcy >11 µmol/L, ranging from 11.5 to 16 µmol/L. tHcy results did not differ from the NHANES data (NHANES results for males: 6.2 µmol/L for non-Hispanic Caucasian, 6.4 for non-Hispanic African American, and 6.4 for Mexican American; females: 5.8, 6,1 and 5.5 respectively, 95% CIs range from 5.2 – 6.7). tHcy in the 90 children with MTHFR c.677C > T genetic testing was similar to the larger group with amedian of 5.2 µmol/L (range: <2–11.6), and tHcy was >11 µmol/L in two of the 90 children (prevalence: 2.2%). tHcy was significantly lower among infants less than 12 months (median: 4.6, range: <2–8.3) compared to children 12 months to 21 years (median: 5.4, range: <2–11.6); P = 0.01. In addition, tHcy was higher in adolescents 13–21 years (median 6.1, range 3.8–11.6) in comparison to children 1–12 years (median 4.5, range 2.3–9.1); P < 0.01. A statistically significant difference was observed in tHcy between VTE and AIS groups (median: 5.7 [range: <2–11.6] vs. 4.8 [<2–8.9], respectively; P = 0.03), consistent with the difference in ages of the two study populations. Although there was an observed difference in tHcy between those with an idiopathic event versus those with an identified risk factor (median: 4.9, range [<2–8.3] vs. 5.5 [<2–11.6]), the difference was not statistically significant (P = 0.07) and the direction of effect was opposite that expected for thrombophilia (Fig. 2).
Fig. 2.
Mean homocysteine levels in children with thrombosis and controls. The mean tHcy levels in male and female pediatric patients with thrombosis are shown to closely mirror the NHANES healthy US controls.
Frequency of MTHFR c.677C > T variant
The prevalence of heterozygous MTHFR c.677C > T was 33% and of homozygous c.677C > T or compound heterozygous c.677C > T/c.1298A > C was 13%, and the overall c.677C > T allele frequency was 27%. The frequency of c.677C > T did not differ between the VTE and AIS groups (26% vs. 40%, P = 0.14). Although not all children were tested for the c.1298A > C polymorphism, in those tested, the frequency of c.677C > T homozygotes/compound heterozygotes did not differ between the VTE and AIS groups (14% vs. 13%, P = 0.87). Additionally, c.677C > T frequencies did not differ based on age at presentation, presence of kidney disease, whether the event was idiopathic, or the presence of genetic thrombophilia (p > 0.05 for each except where non applicable for cell numbers of 0).
Distribution of tHcy in MTHFR c.677C > T vs. wild-type
tHcy levels did not differ between c.677C > T heterozygotes and those with wild type alleles (median: 5.3 µmol/L [range: 2.4–10.2] vs. 5.3 [<2–10.2], P = 0.86). There was also no observed difference in the median tHcy levels of those with homozygous c.677C > T or compound heterozygotes (4.3 µmol/L [<2–10.5]) compared to wild type (5.3 [<2–10.2], P = 0.18).
Outcomes
The relationship of tHcy level and MTHFR genotype to clinical outcomes was evaluated. Neither tHcy levels nor MTHFR c.677C > T frequency predicted outcomes of progression, recurrence or PTS. Three VTE patients (7%) had progression, while 10 (12% of) VTE (n = 8) and AIS (n = 2) patients had recurrent thromboembolism. The proportion of children with progression and/or recurrence was 23% in the group with homozygous or compound heterozygous mutations in the MTHFR gene and 12% in the group without these mutations. Because the size of the population with progression and/or recurrence was small, in order to determine that compound heterozygous and homozygous MTHFR genotypes were not related to progression and/or recurrence, an odds ratio was used to estimate a sample size that would be needed to reach statistical significance with a power of 80% and an α error of 0.05: a sample size of 250 patients each with and without progression and/or recurrence would be required to achieve statistical significance. Of the 25 patients with limb DVT, 20 (80%) were assessed for PTS. Among these 20 patients, 10 (50%) had some PTS, including 5 (25%) with clinically significant PTS. The proportion of patients with any or functionally significant PTS was too small for the estimation of population size for a power calculation.
The mean tHcy level in patients with progression/recurrence was 5.64 (SD 2.2) compared with a mean of 4.7 (SD 2.24) without progression/recurrence. In order to determine statistically that tHcy does not differ between these two groups with 80% power and an α error of 0.05, 88 subjects would be required in each group. Mean tHcy was 7.16 (SD 1.37) in the patients with functionally significant PTS and 6.46 (SD 2.54) without functionally significant PTS. In order to determine a statistically significant difference in tHcy between these two groups with a power of 80% and an α error of 0.05, 131 subjects per group would be required.
Discussion
The present findings indicate that hyperhomocysteinemia was rare (observed prevalence, 2.4%) in an institution-based prospective cohort of U.S. children with VTE or AIS who receive folate and B12 supplementation in food. This was a lower prevalence of elevated tHcy as compared to the recent study of 141 US children by Nahar et al., which observed an 11% prevalence of hyperhomocysteinemia [16]. It is notable that in the study by Nahar, elevations in homocysteine were determined prior to grain supplementation with folate and B12, whereas results fell following institution of supplementation in 1996. Similarly to that study, a 30% prevalence of tHcy elevated above the 90% percentile was reported in 163 German children with thromboembolism[17]; Germany also does not fortify grain products. We determined lower tHcy in infants <12 months and a trend toward increased tHcy with age; this trend was consistent with the NHANES measurement of tHcy in healthy U.S. children, which found that tHcy increased according to each age quartile [10].
We observed aMTHFR c.677C > T allele frequency of 27%,with a 7.8% prevalence of homozygosity, similar to the overall and homozygosity frequency of 30.8 and 9.7% found in healthy adults in the NHANES database [18], indicating that the MTHFR c.677C > T variant is not more prevalent in this population with thrombotic events, in contrast to the report of Rook and colleagues [19].
Although previous studies - particularly those conducted in Europe - have suggested that the MTHFR c.677C > T variant increases the risk of mild elevations of tHcy, we observed no relationship between tHcy and C667T status in our US VTE and AIS study population [20,21]. The lack of association between tHcy and the MTHFR c.677C > T genotype in our study is supported by work by Kluijtmans et al.,whose study concluded that genetics account for only 9% of tHcy, whereas diet plays a much larger role [22]. The Kluijtmans study attributed 35% of variability in tHcy to vitamin B12 and folate levels, which is concordant with the hypothesis that folate supplementation of grain products in the U.S. mitigates the influence of MTHFR c.677C > T on tHcy. Additionally, we detected no relationship of tHcy or MTHFR c.667C > T status to VTE and AIS outcomes, including recurrent thromboembolism and development of PTS (the latter among limb VTE patients), although adverse outcomes in this population were infrequent events and sample sizes of 88 to 250 subjects with and without adverse outcomes would be needed for statistical power.
Although hyperhomocysteinemia was rare in this pediatric population, there is some debate as to the significance of a single tHcy value, particularly if not obtained immediately preceding or following the thrombotic event, as it may or may not be reflective of the tHcy at the time of the event. Extremely elevated values (greater than 50 µmol/L) are likely due to inborn errors of metabolism or severe vitamin B12 deficiency and would require expert consultation and diagnosis. However, a study following the weekly variability of tHcy levels in adults demonstrated a reliability coefficient of 0.94 at 4 weeks and 0.82 at 30 months (excluding one outlier), indicating that tHcy is fairly constant over at least a month, and a single value may be representative for up to 30 months [23].
Our findings call into question the utility of the widespread practice of MTHFR c.677C > T mutation testing in pediatric patients with thromboembolic disease. Following clinical screening, all patients with indications of metabolic disease, known malabsorption syndromes or specific indicators of cystathionine B-synthase deficiency such as near-sightedness, ectopic lenses, tall stature or bone abnormalities should have screening with tHcy, as recent data suggest that some highly responsive variants of cystathionine B-synthase deficiency may have thrombotic- only presentations and are much more common in the population than previously realized [24]. We acknowledge the importance of homocysteine testing to detect mild to moderate hyperhomocysteinemia resulting from other gene mutations as well as the low cost of this assay.
Limitations of the present study include a relatively small population, and the small subgroup of patients with recurrence, progression or PTS, for which the study was inadequately powered for statistical significance. In addition, timing for the collection of non-fasting homocysteine samples (relative to incident acute thromboembolism) was variable. Notwithstanding these limitations, the findings of this study indicate that the MTHFR c.677C > T variant is not a strong risk factor for incident VTE or AIS in U.S. children, and may not influence outcomes of recurrent thromboembolism or PTS in children with a first thromboembolic event. In addition, elevations of tHcy are uncommon in US children and are not related to common MTHFR polymorphisms. Therefore, clinical decisions based on MTHFR c.677C > T genotypes or mildly elevated tHcy should be made cautiously. Future work should examine a larger group of children prospectively to assess for the possibility of any dose–response in incidence VTE/AIS risk or risk of adverse thromboembolic outcomes within the normal tHcy range, which cannot be fully excluded in the present study. We suggest that routine testing of MTHFR c.677C > T genotype as part of a thrombophilia evaluation in children with incident thromboembolism is not warranted until larger studies have been performed in order to establish or refute a link between MTHFR and adverse outcomes.
Abbreviations
- tHcy
total plasma homocysteine level
- VTE
venous thromboembolism
- AIS
arterial ischemic stroke
- MTHFR c.677C > T
Methylenetetrahydrofolate Reductase
- PTS
post thrombotic syndrome.
Footnotes
Authorship Contributions
Emily Joachim made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data; Ms. Joachim wrote the initial draft of this manuscript, as well as each further draft and approved the submitted version.
Neil A. Goldenberg made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data; Dr. Goldenberg contributed to each draft of this manuscript and approved the submitted version.
Dr. Timothy Bernard made substantial contributions to acquisition of data, interpretation of data, critique of each draft and approval of the submitted version.
Dr. Jennifer Armstrong-Wells made substantial contributions to acquisition of data, interpretation of data, critique of each draft and approval of the submitted version.
Dr. Sally Stabler made substantial contributions to conception and design, and interpretation of data; Dr. Stabler read each draft of the manuscript, made important contributions regarding the importance of tHcy in the detection of metabolic disorders and approved the submitted version.
Marilyn Manco-Johnson made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data; as well as primary editor of the first draft; Dr.Manco-Johnson approved each version of the manuscript including the submitted version.
Conflict of Interest Statement
None.
References
- 1.McCully K. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56:111–128. [PMC free article] [PubMed] [Google Scholar]
- 2.Undas A, Brozek J, Szczeklik A. Homocysteine and thrombosis: from basic science to clinical evidence. Thromb Haemost. 2005;94:907–915. doi: 10.1160/TH05-05-0313. [DOI] [PubMed] [Google Scholar]
- 3.Mudd SH, Skovby F, Levy HL, Pettigrew KD, Wilcken B, Pyeritz RE, et al. The natural history of homocystinuria due to cystathionine beta-synthase deficiency. Am J Hum Genet. 1985;37:1–31. [PMC free article] [PubMed] [Google Scholar]
- 4.den Heijer M. Hyperhomocysteinaemia as a risk factor for venous thrombosis: an update of the current evidence. Clin Chem Lab Med. 2003;41:1404–1407. doi: 10.1515/CCLM.2003.215. [DOI] [PubMed] [Google Scholar]
- 5.Jacques P, Selhub J, Bostom A, Wilson P, Rosenberg I. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med. 1999;340:1449–1454. doi: 10.1056/NEJM199905133401901. [DOI] [PubMed] [Google Scholar]
- 6.den Heijer M, Willems HP, Blom HJ, Gerrits WB, Cattaneo M, Eichinger S, et al. Homocysteine lowering by B vitamins and the secondary prevention of deep vein thrombosis and pulmonary embolism: A randomized, placebo-controlled, double-blind trial. Blood. 2007;109:139–144. doi: 10.1182/blood-2006-04-014654. [DOI] [PubMed] [Google Scholar]
- 7.Frosst P, Blom H, Milos R, Goyette P, Sheppard C, Matthews R, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 8.Den Heijer M, Lewington S, Clarke R. Homocysteine, MTHFR C.677C > T and risk of venous thrombosis: a meta-analysis of published epidemiological studies. J Thromb Haemost. 2005;3:292–299. doi: 10.1111/j.1538-7836.2005.01141.x. [DOI] [PubMed] [Google Scholar]
- 9.Bezemer I, Doggen C, Vos H, Rosendaal F. No association between the common MTHFR C.677C > T 677C- > T polymorphism and venous thrombosis: results from the MEGA study. Arch Intern Med. 2007;167:497–501. doi: 10.1001/archinte.167.5.497. [DOI] [PubMed] [Google Scholar]
- 10.Pfeiffer CM, Caudill SP, Gunter EW, Osterloh J, Sampson EJ. Biochemical indicators of B vitamin status in the US population after folic acid fortification: results from the National Health and Nutrition Examination Survey 1999–2000. Am J Clin Nutr. 2005;82:442–450. doi: 10.1093/ajcn.82.2.442. [DOI] [PubMed] [Google Scholar]
- 11.Goldenberg NA, Durham J, Knapp-Clevenger R, Manco-Johnson MJ. A thrombolytic regimen for high –risk deep vein thrombosis may substantially reduce the risk of postthrombotic syndrome in children. Blood. 2007;110:45–53. doi: 10.1182/blood-2006-12-061234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldenberg NA, Pounder E, Knapp-Clevenger R, Manco-Johnson MJ. Validation of Upper Extremity Post-Thrombotic Syndrome Outcome Measurement in Children. J Pediatr. 2010;5:852–855. doi: 10.1016/j.jpeds.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldenberg NA, Branchford B, Wang M, Ray C, Durham J, Manco-Johnson MJ. Percutaneous Mechanical and Pharmacomechanical Thrombolysis for Occlusive Deep Vein Thrombosis of the Proximal Limb in Adolescent Subjects: Findings from an Institution-based Prospective Inception Cohort Study of Pediatric Venous Thromboembolism. J Vasc Interv Radiol. 2011;22:121–132. doi: 10.1016/j.jvir.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stabler SP, Marcell PD, Podell ER, Allen RH, Savage DG, Lindenbaum J. Elevation of total homocysteine in the serum of patients with cobalamin or folate deficiency detected by capillary gas chromatography–mass spectrometry. J Clin Invest. 1988 Feb;81(2):466–474. doi: 10.1172/JCI113343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martens GA, DeNayer J, DeSmet D, Couck P, Gorus F, Gerlo E. Homocysteine measurement by Vitros Microtip homocysteine assay. Clin Chem Lab Med. 2008;46:283–286. doi: 10.1515/CCLM.2008.054. [DOI] [PubMed] [Google Scholar]
- 16.Nahar A, Sabo C, Chitlur M, Ravindranath Y, Lusher J, Rajpurkar M. Plasma homocysteine levels, methylene tetrahydrofolate reductase polymorphisms, and the risk of thromboembolism in children. J Pediatr Hematol Oncol. 2011;33:330–333. doi: 10.1097/MPH.0b013e318219324f. [DOI] [PubMed] [Google Scholar]
- 17.Kosch A, Koch HG, Heinecke A, Kurnik K, Heller C, Nowak-Gottl U for the Childhood Thrombophilia Study Group. Increased fasting total homocysteine plasma levels as a risk factor for thromboembolism in children. Thromb Haemost. 2004;91:308–314. doi: 10.1160/TH03-02-0038. [DOI] [PubMed] [Google Scholar]
- 18.Chang MH, Lindegren ML, Butler MA, Chanock SJ, Dowling NF, Gallagher M, et al. Prevalence in the United States of selected candidate gene variants: Third National Health and Nutrition Examination Survey, 1991–1994. Am J Epidemiol. 2009;169:54–66. doi: 10.1093/aje/kwn286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rook J, Nugent D, Young G. Pediatric stroke and methylenetetrahydrofolate reductase polymorphisms: an examination of C677T and A1298C mutations. J Pediatr Hematol Oncol. 2005;27:590–593. doi: 10.1097/01.mph.0000188119.33452.fd. [DOI] [PubMed] [Google Scholar]
- 20.Castro R, Rivera I, Ravasco P, Jakobs C, Blom H, Camilo M, et al. 5,10-Methylenetetrahydrofolate reductase 677C– > T and 1298A– > C mutations are genetic determinants of elevated homocysteine. QJM. 2003;96:297–303. doi: 10.1093/qjmed/hcg039. [DOI] [PubMed] [Google Scholar]
- 21.Cardo E, Monrós E, Colomé C, Artuch R, Campistol J, Pineda M, et al. Children with stroke: polymorphism of the MTHFR C.677C > T gene, mild hyperhomocysteinemia, and vitamin status. J Child Neurol. 2000;15:295–298. doi: 10.1177/088307380001500505. [DOI] [PubMed] [Google Scholar]
- 22.Kluijtmans L, Young I, Boreham C, Murray L, McMaster D, McNulty H, et al. Genetic and nutritional factors contributing to hyperhomocysteinemia in young adults. Blood. 2003;101:2483–2488. doi: 10.1182/blood.V101.7.2483. [DOI] [PubMed] [Google Scholar]
- 23.Garg UC, Zheng ZJ, Folsom AR, Moyer YS, Tsai MY, McGovern P, et al. Short-term and long-term variability of plasma homocysteine measurement. Clin Chem. 1997;43:141–145. [PubMed] [Google Scholar]
- 24.Jansosik M, Sokolova J, Janosikova B, Krit J, Klatovska V, Kozich V. Birth Prevalence of Homocystinuria in Central Europe: Frequency and Pathogenicity of Mutation c.1105C > T (p.R369C) in the Cystathionine Beta-Synthase Gene. J Pediatr. 2009;154:431–437. doi: 10.1016/j.jpeds.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]