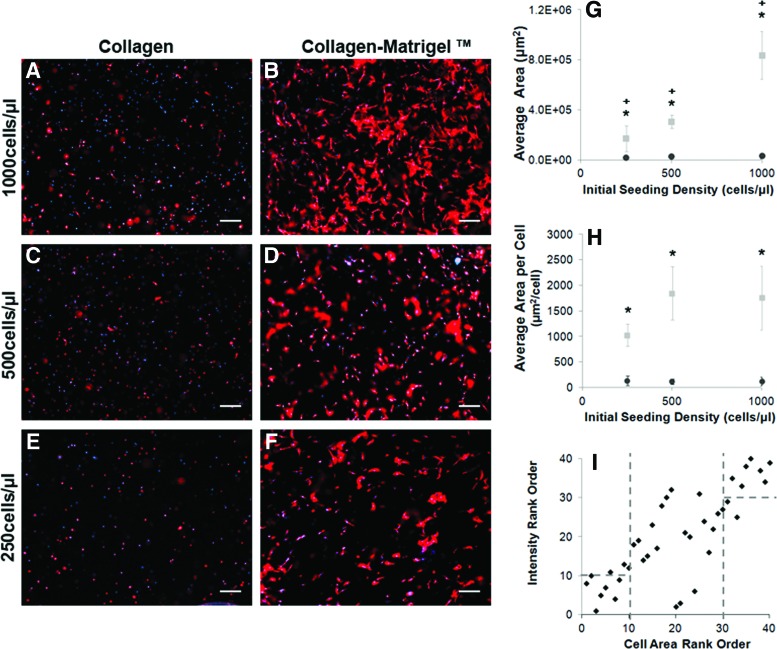
FIG. 2.
Traditional microscopy confirms RAMP analysis of Schwann cell response to model biomaterials. Arrays were imaged using microscopy and changes in cell morphology were analyzed. Schwann cells were stained with phalloidin to visualize the actin cytoskeleton (red) and the nuclei were labeled with DAPI (blue). Cells were seeded within the collagen (A, C, E) or collagen–Matrigel (B, D, F) biomaterials at densities ranging from 250 to 1000 cells per μL and were cultured for 3 days. Schwann cells remained largely rounded in the collagen-only construct, while the collagen–Matrigel biomaterial supported a spread morphology at all cell densities examined. (G) Using ImageJ, cell area was significantly (*p<0.05, n=3, m=12) higher for Schwann cells cultured within collagen–Matrigel (gray squares) over those cultured within collagen (black circles). As expected, there was a significant increase in the overall cell area with increasing seeding density (+p<0.05, n=3, m=12). (H) Normalizing the cell area to cell number provides the average spreading area per cell, which was shown to be significantly (*p<0.05, n=3, m=12) higher for cells seeded within collagen–Matrigel (gray squares) than Schwann cells cultured in collagen (black circles). There was not a significant difference in spreading area per cell between seeding densities when normalized to cell number. (I) High-throughput, low-resolution scanner intensity measurements from RAMP correlate with low-throughput, higher resolution microscopy measurements for samples with varying seeding density, matrix, and duration with few outliers. “Hits” found in the scanned image show comparable correlation to the samples with the highest Schwann cell spreading area. Error bars represent one standard deviation. Scale bar=200 μm. 4×magnification. DAPI, 4′,6-diamidino-2-phenylindol. Color images available online at www.liebertpub.com/tec