Abstract
Objective
The interaction of protein C (PC) with the endothelial protein C receptor (EPCR) enhances activated PC (APC) generation. We performed targeted gene sequencing of the PC gene (PROC) and EPCR genes (PROCR) in patients with unprovoked venous thromboembolism (VTE) to determine if mutations that impair PC-EPCR interactions are associated with an increased risk of VTE.
Approach and Results
We sequenced exon 3 of PROC and exons 2 and 3 of PROCR (the exons that encode the protein-protein binding domains of PC and EPCR) in 653 patients with unprovoked VTE and in 627 healthy controls. Five single nucleotide variants (SNVs), each in individual patients, were identified that result in abnormal PC (Arg9Cys, Val34Met, Arg-1Cys) or abnormal EPCR proteins (Arg96Cys and Val170Leu). None of these SNVs were found in the controls. When the PC variants were expressed in human embryonic kidney (HEK) 293 cells, all exhibited decreased synthesis, and two of the variants had reduced capacity for APC generation. When expressed on the surface of HEK293 cells, the EPCR variants showed reduced affinity for fluorescently-labeled PC. In addition, the previously reported EPCR A3 haplotype, which promotes cellular “shedding” of EPCR, is overrepresented in the patient group (P=0.001).
Conclusions
This is the first targeted DNA sequencing analysis of PROC and PROCR in a large group of patients with unprovoked VTE. Our data suggest that mutations that impair PC-EPCR interactions may be associated with an increased risk of VTE.
Keywords: Protein C, endothelial protein C receptor (EPCR), venous thromboembolism, gene sequencing, polymorphisms
INTRODUCTION
The protein C (PC) pathway plays a major role in inhibiting blood coagulation. PC is activated on the surface of vascular endothelial cells by the thrombin-thrombomodulin (TM) complex. The endothelial protein C receptor (EPCR), a receptor which binds circulating PC and presents it to the thrombin-TM complex, enhances PC activation by ~8-fold in vitro1 and by ~20-fold in vivo2. Activated PC (APC), in conjunction with its cofactor protein S, degrades coagulation cofactors Va and VIIIa, thereby attenuating thrombin generation.
Hereditary thrombophilia is identified in about one-third of patients with unprovoked venous thromboembolism (VTE)3. Most hereditary thrombophilic defects are related to loss of function of anticoagulant proteins (e.g. deficiencies of PC, protein S, or antithrombin) or gain of function in procoagulant factors (e.g. increased levels of prothrombin, factor VIII or other coagulation factors) 4,5. Family history is a strong risk factor for VTE suggesting that many more patients with unprovoked VTE have as yet unidentified genetic abnormalities that predispose them to thrombosis. Identification of these abnormalities is important because it enables more comprehensive counseling of patients and family members, including more vigilant use of VTE prophylaxis in high risk situations (e.g. prolonged immobility peri-operatively) and avoidance of estrogen therapy6,7. Presence of thrombophilia, if it were a strong predictor of recurrent VTE, could also influence the duration of anticoagulation therapy.
PC deficiency is generally subdivided into two types. Type I deficiencies are characterized by decreases in PC antigen, whereas type II deficiencies are characterized by abnormal biologic activity. Abnormal PC activity and can be assessed by amidolytic assays (which can detect abnormalities in the serine protease domain of PC)8, and by coagulation tests such as the APTT. In both the amidolytic and clotting assays, the PC is commonly converted to APC by protac, an activator found in snake venom9,10. In vivo, however, the conversion of PC to APC is accelerated by the binding of PC to EPCR on the vascular endothelial cell surface11. Thus, genetic mutations that impair PC-EPCR interactions would escape detection with currently available diagnostic assays.
PC contains a Gla domain (which contains nine γ-carboxylated glutamic acid residues), a connecting region, two EGF-like domains, an activation peptide, and the serine protease domain12,13. According to the crystal structure, the majority of PC residues contributing to EPCR binding are located on the ω-loop of the Gla domain14 (which is encoded by exon 3 of the PC gene). EPCR consists of an α1 and α2 domain which form a ligand binding groove composed of two antiparallel α-helices that sit upon an 8-stranded β-sheet platform14. Mutagenesis studies of EPCR and the EPCR crystal structure have shown that the PC binding domain is located at the distal end of the two α-helical segments14,15 (encoded by exons 2 and 3 of the EPCR gene).
We hypothesized that mutations that impair PC-EPCR interactions may be associated with an increased risk of venous thromboembolism (VTE). To explore this possibility, we sequenced the PC gene (PROC) and EPCR genes (PROCR) in 657 patients with unprovoked VTE (from the ELATE trial which compared low-intensity and conventional-intensity anticoagulation with warfarin for the prevention of recurrent VTE16) and in 627 controls with no known history of VTE. We focused our DNA sequencing on the following regions: (a) exon 3 of PROC (which encodes for the Gla domain of PC, the region of PC that binds to EPCR), (b) exons 2 and 3 of PROCR (which encode the PC-binding domain of EPCR), and (c) exon 4 of PROCR which contains the previously reported EPCR A3 haplotype polymorphism site. The EPCR A3 haplotype is associated with reduced EPCR function due to increased endothelial shedding of EPCR17,18. This study is the first targeted DNA gene sequencing analyses of the PROC and PROCR genes in a large group of patients with unprovoked VTE.
MATERIALS AND METHODS
The study design and experimental methods are described in detail in the online-only Supplement.
Our study included 653 patients with unprovoked VTE and 627 control subjects with no history of thromboembolic disease.
RESULTS
A total of 653 patients with unprovoked VTE were included in this study (mean age of 57 ± 15 years and 45% were female). VTE had occurred once in 27%, twice in 51%, more than twice in 22%, and the most recent episode of VTE was proximal deep vein thrombosis only in 66% and pulmonary embolism (with or without deep vein thrombosis) in 34%16. The 627 controls who never had VTE (described in the Methods section) had a mean age of 42 ± 9 years and 49% were female.
To analyze the EPCR-binding domain of PC, we sequenced a 650 bp fragment of PROC which codes for the Gla domain of PC. A list of all single nucleotide variants (SNVs) or single nucleotide polymorphisms (SNPs) found in this study are summarized in Table 1 according to the Human Genome Variation Society (HGVS) nomenclature19. We found 6 SNVs in the 650 bp fragment encompassing the coding region of the Gla domain of PC (Table 2). The first was a C to T transition at nucleotide (nt) 2896 which results in substitution of Arg with Cys at residue −1 in the Gla domain of PC (amino acids are numbered from the N-terminus of the mature secreted protein). The second SNV was a C to T transition at nt 2923 which results in an Arg9Cys substitution in the Gla domain of protein C. The third SNV was a G to A transition at nt 2998 which results in a Val34Met substitution in the Gla domain of protein C. The remaining SNVs were located in intron 2 (C2633G, C2730T). None of these SNVs was present in the controls. We also found common SNPs in intron 3 of the protein C gene in both the patients and the controls.
Table 1.
Human Genome Variation Society (HGVS) Nomenclature of Single Nucleotide Variants (SNVs) in PROC and PROCR
| PROC | |||||
|---|---|---|---|---|---|
| SNV | Consequence | HGVS Designation | Consequence | Mutation database | |
| Exon 3 | C 2896 T | Arg-1Cys | NM_000312.3:c.124C>T | p.Arg42Cys | HGMD CM930604, ProCMD Variant # 13 |
| Exon 3 | C 2923 T | Arg9Cys | NM_000312.3:c.151C>T | p.Arg51Cys | HGMD CM950976, ProCMD Variant # 18 |
| Exon 3 | G 2998 A | Val34Met | NM_000312.3:c.226G>A | p.Val76Met | HGMD CM920593, ProCMD Variant # 29 |
| Intron 2 | C 2633 G |
NM_000312.3:c.70+1061C>G NM_000312.3:c.71-210C>G |
|||
| Intron 2 | C 2730 T |
NM_000312.3:c.70+1158C>T NM_000312.3:c.71-113C>T |
|||
| Intron 3 | G 3310 A | NM_000312.3:c.237+301G>A | |||
| PROCR | |||||
|---|---|---|---|---|---|
| SNV or insertion | Consequence | HGVS Designation | Consequence | Mutation database | |
| Exon 3 | C 6367 T | Arg96Cys | NM_006404.3:c.337C>T | p.Arg113Cys | NCBI SNP database rs146420040 |
| Exon 3 | G 6589 C | Val170Leu | NM_006404.3:c.559G>C | p.Val187Leu | NCBI SNP database rs61731003 |
| Exon 3 | C 6519 T | Phe to Phe | NM_006404.3:c.489C>T | p.Phe163Phe | Silent Mutation |
| Exon 3 | 23bp insertion at 6367 | Insertion codes for 5 amino acids (YPQFL) followed by a stop codon | NM_006404.3:c.337_359insTATCCACAGTTCCTCTGACCATC | ||
| Intron 2 | G 5212 A | NM_006404.3:c.322 + 77G>A | |||
| Intron 2 | G 5195 A | NM_006404.3:c.322 + 60G>A | |||
| Intron 2 | C 5334 A | NM_006404.3:c.322 + 199C>A | |||
| Intron 3 | A 6668 T | NM_006404.3:c.601 + 37A>T | |||
Table 2.
Summary of SNVs identified in Exon 3, Intron 2, and Intron 3 of PROC
| SNV | Location | Consequence | # in patients (n=653) | # in controls (n=627) |
|---|---|---|---|---|
| C 2896 T | Exon 3 | Arg to Cys at position −1 | 1 | 0 |
| C 2923 T | Exon 3 | Arg9Cys | 1 | 0 |
| G 2998 A | Exon 3 | Val34Met | 1 | 0 |
| C 2633 G | Intron 2 | 1 | 0 | |
| C 2730 T | Intron 2 | 1 | 0 | |
| G3310A | Intron 3 | G/G 430 (65.9%) G/A 193 (30.0% A/A 30 (4.6%) |
G/G 395 (63%) G/A 210 (33.5%) A/A 22 (3.5%) |
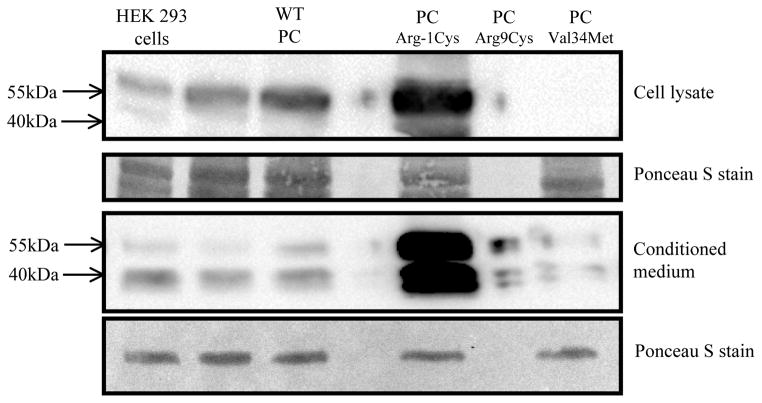
Using stable transfections, we expressed wildtype human PC (WT PC) as well as the three PC variants (PCArg-1Cys, PCArg9Cys, PCVal34Met) in HEK293 cells. All cell lines contained similar levels of PC mRNA as assessed by real-time PCR quantitation (the PCArg-1Cys, PCArg9Cys, and PCVal34Met cell lines contained 57%, 172%, and 134%, respectively, of mRNA relative to the WT PC cell line). The cell lysates and conditioned medium were then subjected to Western blot analysis. As shown in figure 1, WT PC was detected in the cell lysate as well as in the conditioned medium. The two PC bands in the conditioned medium are consistent with the α- and β-forms of PC, which differ in glycosylation state at Asn32920. In contrast, PCArg-1Cys, PCArg9Cys, and PCVal34Met were detected at lower levels in the cell lysates and in the conditioned medium.
Figure 1. Western Blot analysis of PC variants expressed in HEK293 cells.
HEK293 cells were stably transfected with cDNAs encoding either wildtype PC or PC variants (PCArg-1Cys, PCArg9Cys, PCVal34Met). All cell lines contained similar levels of PC mRNA as assessed by real-time PCR quantitation The cell lysates and conditioned medium were separated by SDS-PAGE (4–15% gradient gel) under reducing conditions, and subjected to Western blot analysis with HPC4, a monoclonal antibody against human PC. The nitrocellulose membrane was stained with Ponceau S dye to confirm that similar amounts of protein were loaded in each lane (sample section of the nitrocellulose membrane is shown).
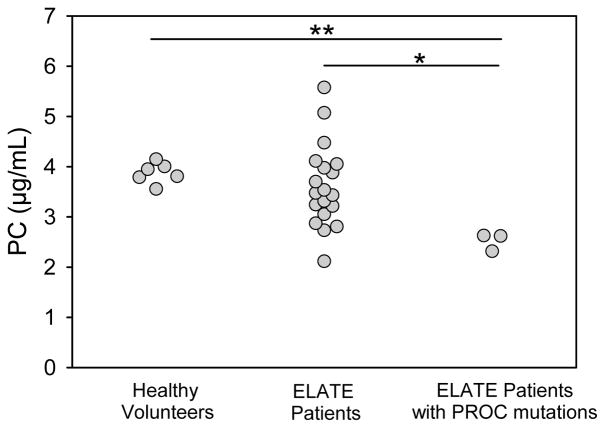
To determine if these PROC mutations result in a type I PC deficiency, we measured PC antigen levels in 21 patients with VTE (3 patients had mutations in the Gla domain of the PROC gene, and 18 did not). We also measured PC antigen levels in 6 healthy volunteers. As shown in figure 2, the mean PC antigen level in the VTE patients without the PROC Gla domain mutations (3.61 ± 0.81 μg/mL) was similar to that in the healthy volunteers (3.88 ± 0.19 μg/mL) (P=0.19). In contrast, the mean PC antigen level in the VTE patients with the PROC Gla domain mutations (2.52 ± 0.15 μg/mL) was lower than that in healthy volunteers (P<0.001) and in the VTE patients without the PROC mutations (P=0.037). The PC antigen levels in the patients harboring the Val34Met, Arg9Cys, and Arg-1Cys mutations was 66%, 66%, and 58% of normal levels, respectively, suggesting that these mutations result in a heterozygous type 1 deficiency.
Figure 2. PC antigen levels in healthy volunteers and in the ELATE Study patients (with or without PROC Gla domain mutations).
PC antigen levels were measure in plasma samples from 6 healthy volunteers and in 21 ELATE Study patients (3 patients had mutations in the Gla domain of PC and 18 did not). * Denotes P<0.05; **denotes P<0.001.
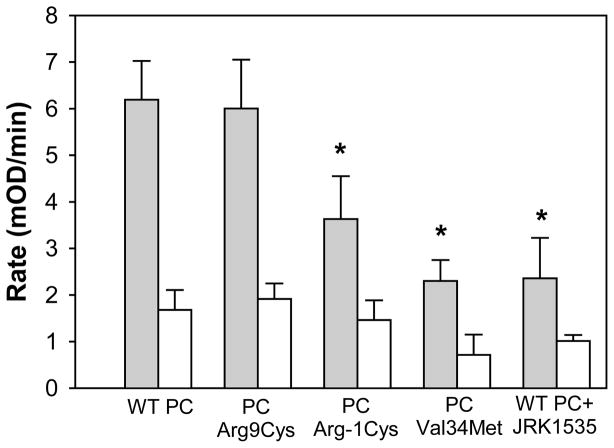
Given that the PROC mutations are located in the Gla domain of PC, it is also possible that these mutations result in a heterozygous type IIb deficiency due to impaired ability to bind to EPCR (ie. normal amidolytic activity but reduced APC anticoagulant activity). To explore this possibility, the PC variants were purified from the conditioned medium of the stable cell lines and subjected to PC activation assays. As shown in figure 3, the activation rate of WT PC is about 4-fold higher on HEK293 cells expressing EPCR and TM (gray bars) compared with that observed on HEK293 cells (white bars). Pre-incubation of WT PC with an anti-EPCR antibody that blocks PC-EPCR interactions (JRK 1535, 500 nM) inhibits the activation rate of WT PC on HEK293/EPCR/TM cells. The activation rate of PCArg9Cys is similar to that of WT PC, whereas PCArg-1Cys and PCVal34Met have impaired capacity to generate APC. Thus, patients carrying the PCArg-1Cys and PCVal34Met mutations have a type I as well as a type IIb heterozygous PC deficiency.
Figure 3. Analysis of WT PC and PC variants in PC activation assays.
PC activation assays were performed as described under “Materials and Methods”. Gray bars, PC activation rates in the presence of HEK293 cells expressing EPCR and TM. White bars, PC activation rates in the presence of HEK293 cells. JRK1535 (an antibody that inhibits the binding of PC to EPCR) was used at a concentration of 500 nM. The bars represent the mean, and the lines above the bars reflect the S.E. of at least three determinations. * denotes P<0.05 compared with WT PC.
To analyze the PC-binding domain of EPCR, we sequenced exons 2 and 3 of PROCR which encode for the PC-binding domain of EPCR. PCR amplification and sequencing of exons 2 and 3 was successful in all controls and in 630/653 and 649/653 of the patients, respectively. No SNVs were detected in exon 2 of PROCR in either the patients or in the controls. As shown in Table 3, we found three SNVs in exon 3 that occurred in the patients but not in the controls. The first SNV was a C to T transition at nt 6367 which results in a Arg96Cys substitution. The second SNV was a G to C transition at 6589 which results in a Val170Leu substitution. The third SNV was a silent mutation (C6519T). Within intron 2, we found three SNVs (G5212A, G5195A, C5334A) in the patients but not in the controls. Within intron 3, we found a SNV (G deletion at 6738) that is present in both the patients and controls, and a SNV (A6668T) that is present at a higher frequency in the patients than in the controls.
Table 3.
Summary of SNVs identified in Exon 3, Intron 2, and Intron 3 of the PROCR
| SNV | Location | Consequence | # in patients | # in controls |
|---|---|---|---|---|
| C 6367 T | Exon 3 | Arg96Cys | 1 | 0 |
| G 6589 C | Exon 3 | Val170Leu | 1 | 0 |
| C 6519 T | Exon 3 | Silent (Phe to Phe) | 1 | 0 |
| G 5212 A | Intron 2 | 2 | 0 | |
| G 5195 A | Intron 2 | 1 | 0 | |
| C 5334 A | Intron 2 | 2 | 0 | |
| A6668T | Intron 3 | 7 | 2 | |
| G deletion @6738 | Intron 3 | 1 | 1 |
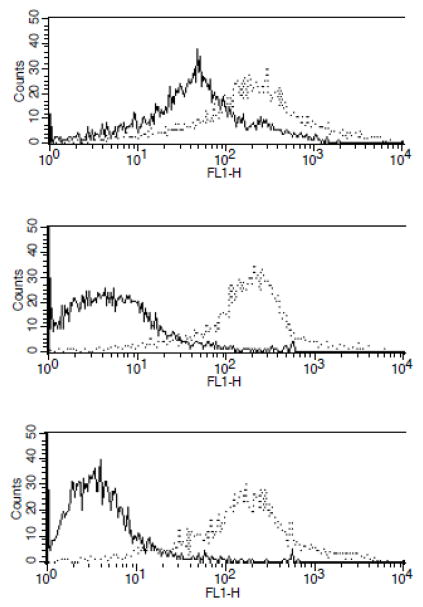
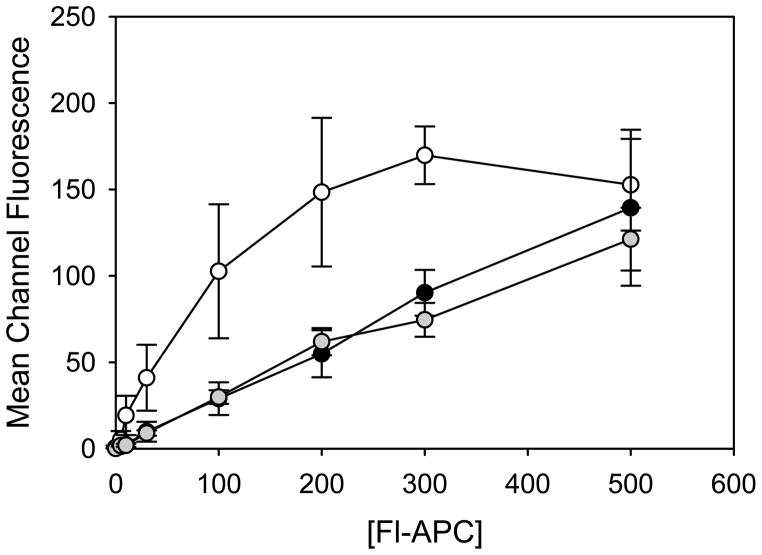
To determine if the EPCR SNVs result in abnormal proteins with reduced binding affinity for PC, the cDNAs of WT EPCR and the two EPCR variants were stably transfected into HEK293 cells, and the ability of the EPCR variants to bind to fluorescently-labelled APC (Fl-APC) was measured by flow cytometry. All three stable cell lines expressed similar levels of EPCR on the cell surface as confirmed by flow cytometry using an anti-EPCR antibody (JRK 1535) (figure 4). WT EPCR bound to Fl-APC with a Kd value of 77 ± 21 nM, consistent with previous reports15,21. In contrast, EPCR variants Arg96Cys and Val170Leu bound to Fl-APC with reduced affinities (Kd values ≥2 μM, which is higher than the circulating concentration of PC (~60nM). The binding curves obtained from the flow cytometry studies are shown in figure 5.
Figure 4. Binding of EPCR variants to fluorescently-labelled APC (Fl-APC) and FITC-JRK 1535 (anti-EPCR mAb).
HEK293 cells stably expressing wild-type human EPCR or EPCR variants (Arg96Cys and Val170Leu) were incubated at room temperature with 100 nM of either Fl-APC (solid line) or FITC-labelled JRK 1535, a monoclonal antibody against human EPCR (dotted line). Binding of these ligands to the cells was analyzed by flow cytometry. Top panel, wildtype EPCR; middle panel, EPCR Arg96Cys; bottom panel, EPCR Val170Leu.
Figure 5. Binding of Fl-APC to WT EPCR or EPCR variants expressed on HEK293 cells.
The binding of fluorescently-labelled APC (Fl-APC) to EPCR variants was assessed by flow cytometry as described under “Materials and Methods”. The values correspond to the mean and the S.E. of 3 separate experiments. Symbols: WT EPCR (white circles); EPCR Val170Leu (gray circles); EPCR Arg96Cys (black circles).
We also determined the prevalence of the previously reported PROCR A3 haplotype17. This haplotype results in substitution of Ser219 with Gly in the transmembrane domain of EPCR, which leads to increased endothelial shedding of EPCR and reduced EPCR function17. The PROCR A3 haplotype occurred at a significantly higher frequency in the patient group compared with the control group (P=0.001; Table 4). It should be noted that none of the carriers of the PROCR A3 haplotype were carriers of the PC or EPCR coding region SNVs described above.
Table 4.
Frequency of the A3 Haplotype in PROCR
| Non-A3 Genotype | A3 Heterozygotes | A3 Homozygotes | Total A3 Haplotype | |
|---|---|---|---|---|
| ELATE patients (n=657) | 497 (77.1%) | 137 (21.2%) | 11 (1.7%) | 148 (22.9%)* |
| Controls (n=627) | 501 (86.5%) | 72 (12.4%) | 6 (1.0%) | 80 (13.5%) |
P=<0.001 for ELATE patients compared to all controls
Finally, we identified a 23 bp insertion in exon 3 of PROCR in one control individual but not in any of the VTE patients (insertion described in Table 1). Previous studies have shown that this insertion codes for 5 amino acids (YPQFL) which is followed by a stop codon, resulting in an abnormal EPCR protein that lacks part of the extracellular domain, the transmembrane domain, and the cytoplasmic tail22.
DISCUSSION
In this study, we explored the possibility that genetic mutations that impair PC-EPCR interactions may be associated with an increased risk of VTE. With respect to PROC, we identified three SNVs, each in individual patients, that result in mutations in the Gla domain of PC (PCArg-1Cys, PCArg9Cys, PCVal34Met). The most common thrombophilic defects in the ELATE patients were Factor V Leiden, the prothrombin G20210A gene mutation, and antithrombin deficiency, with prevalences of 26.5% 9.3%, and 3.6%, respectively3. It should be noted that none of these three patients were carriers of the Factor V Leiden gene, the prothrombin gene mutations, or had antithrombin deficiency.
All three PROC SNVs have been previously reported (HGMD CM930604; HGMD CM950976; HGMD CM920593). In this study, we showed that the three PC variants displayed reduced protein synthesis when expressed in HEK293 cells (figure 1), and thus are classified as type I deficiencies. Consistent with this observation, the PC antigen levels in the ELATE Study patients harboring the Val34Met, Arg9Cys, and Arg-1Cys mutations are lower than those in healthy volunteers and in the ELATE Study patients without PC mutations (figure 2). Interestingly, the variants are secreted as a lower molecular weight form of approximately 40kDa (figure 1). PC possesses four N-linked glycosylation sites: one is located in the first EGF domain (Asn 97), and three are located in the serine protease domain (Asn248, Asn313, and Asn 329)20. α-PC, which is glycosylated at all four sites, accounts for about 70% of plasma PC. In contrast, β-PC which lacks glycosylation at Asn 329 and thus has a lower molecular weight, accounts for approximately 30% of plasma PC. Previous studies suggest that the degree of glycosylation at Asn 329 is influenced by the correct post-translational processing of the Gla domain23. Thus, the presence of mutations within the Gla domain of the PC variants likely favors the formation of the β-form of PC. In patients, the presence of only the β-form of PC in plasma results in decreased anticoagulant activity24. Specifically, the naturally occurring PCN329T mutation results in an abnormal APC with impaired ability to inactivate Factor Va.
The PCArg-1Cys and PCArg9Cys variants, both of which carry a free cysteine residue, have been shown to form a complex with alpha 1-microglobulin25. Although, we did not observe complexes between these PC variants and alpha 1-microglobulin based on our Western Blot analysis (presumably because HEK293 cells do not synthesize alpha 1-microglobulin), it is possible that these complexes exist in the patient plasma.
We also observed that the PCArg-1Cys and PCVal34Met variants (secreted and purified from HEK293 cells) have reduced capacity to be converted to APC on the surface of TM- and EPCR-expressing cells (figure 3). This is consistent with previous studies which showed that mutation of Arg-1 to Cys in PC abolishes binding to EPCR26. Thus, patients harboring the PCArg-1Cys or PCVal34Met mutation would have a type I as well as a type II heterozygous PC deficiency. Physiologically, however, plasma levels of the PCArg-1Cys and PCVal34Met proteins would be very low, below that of the Kd for PC/EPCR interactions (30 nM)27,28. Therefore, the PCArg-1Cys and PCVal34Met mutations may indirectly result in a type II deficiency due reduced plasma PC levels.
With respect to PROCR polymorphisms in the VTE patients, we identified two SNVs that result in EPCR variants (Arg96Cys and Val170Leu). These SNVs have been previously reported (NCBI rs146420040 and NCBI rs61731003). In the current study, we demonstrated that introduction of either the Arg96Cys or the Val170Leu mutation results in abnormal EPCR protein with impaired ability to bind PC. Residues Arg96 and Val170 are located near residues which form hydrogen bonds between EPCR and Gla residues of PC14,15. Although these EPCR variants do not bind to PC, they retain the ability to bind to JRK1535 (a monoclonal antibody to EPCR) suggesting that the tertiary structure is maintained. In baboons, pretreatment with a monoclonal antibody to EPCR (which blocks PC binding) reduced the amount of thrombin-induced APC generation by 88%2. EPCR gene disruption in mice results in early embryonic lethality due to placental thrombosis29.
Other polymorphisms in PROCR have been described. The first reported abnormality was a 23-base pair insertion in exon 3 which leads to the production of a truncated EPCR which does not bind to PC22. In the present study, we found this insertion in one of our control subjects. However, the clinical impact of this mutation is difficult to assess because of its low allelic frequency22,30–33. More recent studies have shown that soluble EPCR (sEPCR) levels occur with a bimodal distribution in the normal population; ~80% of subjects have low levels of sEPCR (<180 ng/ml) whereas ~20% have high levels (between 200 and 700 ng/ml)34,35. Analysis of PROCR revealed that the A3 haplotype is overrepresented in VTE patients compared with the controls17. The A3 haplotype results in a Ser to Gly substitution in the transmembrane domain of EPCR which promotes cellular shedding of EPCR in endothelial cells36. In the current study, we confirmed that the EPCR A3 haplotype occurs at a significantly higher frequency in the patient group compared with the control group (P<0.001).
There are limitations to our study. First, the probability of finding 5 variants in the cases but not in the controls is P=0.062 (two-tailed Fischer’s exact test). Thus, the results of this study should be validated in larger studies of patients with unprovoked VTE. Second, the control population was younger in age than the ELATE patient. However, the cases and controls do have similar ethnicity and geographical background (predominantly Caucasian) which may prevent false positives due to population-stratification.
To date, no common SNPs in the PROC and PROCR genes have been identified in GWAS studies of VTE37–39. The results of a discovery GWAS for VTE in 1,618 VTE cases of European origin confirmed that well-known association of the FV Leiden variant and the ABO O blood group with VTE37–39. Additional genome-wide significant signals at the F11 region were associated with an increased risk of thrombosis39. Since GWAS studies cannot detect rare variants, the missing heritability for VTE risk may be due to clusters of rare variants. Our study suggests that rare variants in PROC and PROCR are more likely to be found in unprovoked VTE cases than in controls. Our data support the CDRV (Common Disease, Rare Variant) hypothesis that multiple rare variants, with functional effects and relatively high penetrance, are the major contributors to genetic susceptibility to a complex disease such as VTE40,41. Thus, high-throughput sequencing studies of candidate genes may emerge as a more accurate and powerful tool to elucidate the relationship between genetics and VTE.
In summary, this is the first targeted DNA sequencing analysis of PROC and PROCR in a large group of patients with unprovoked VTE. We identified 5 SNVs in the coding regions of PROC and PROCR which are present in the patients but not in the controls. Expression of the mutant proteins revealed impaired synthesis and/or reduced capacity for PC-EPCR interactions. We also validated previous findings that the EPCR A3 “shedding” haplotype is overrepresented in patients with VTE. Our data suggest that genetic mutations that impair PC-EPCR binding interactions may increase the risk of VTE.
SIGNIFICANCE
The endothelial protein C receptor (EPCR) is an important cofactor for the conversion of protein C (PC) to the anticoagulant enzyme activated PC (APC) on the endothelial cell surface. We hypothesized that mutations that impair PC-EPCR interactions may be associated with an increased risk of venous thromboembolism (VTE). We sequenced portions of the PROC and PROCR genes in 653 patients with unprovoked VTE and in 627 healthy controls. Five single nucleotide variants (SNVs) were identified that result in abnormal PC (Arg9Cys, Val34Met, Arg-1Cys) or abnormal EPCR proteins (Arg96Cys and Val170Leu) in the patients. None of these SNVs were found in the controls. Expression of these mutants in HEK293 cells revealed impaired synthesis and/or reduced capacity for PC-EPCR interactions. In addition, the previously reported EPCR A3 haplotype, which promotes cellular “shedding” of EPCR, is overrepresented in the patient group. This is the first targeted DNA sequencing analysis of the PROC and PROCR genes in a large group of patients with unprovoked VTE. Our data suggest that mutations that impair PC-EPCR interactions may be associated with an increased risk of VTE.
Supplementary Material
Acknowledgments
We thank Jenny Nguyen for technical assistance with the DNA sequence analysis in this study.
SOURCES OF FUNDING
This research was supported in part by grant-in-aids from the Heart and Stroke Foundation of Ontario (NA6311 and 00050), by a team grant from the Canadian Institutes of Health Research (MOP-CTP79846), and by an AFP research grant from the Division of Hematology & Thromboembolism at McMaster University.
Footnotes
DISCLOSURES
None.
Reference List
- 1.Stearns-Kurosawa DJ, Kurosawa S, Mollica JS, Ferrell GL, Esmon CT. The endothelial cell protein C receptor augments protein C activation by the thrombin-thrombomodulin complex. Proc Natl Acad Sci U S A. 1996;93:10212–10216. doi: 10.1073/pnas.93.19.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor FB, Jr, Peer GT, Lockhart MS, Ferrell G, Esmon CT. Endothelial cell protein C receptor plays an important role in protein C activation in vivo. Blood. 2001;97:1685–1688. doi: 10.1182/blood.v97.6.1685. [DOI] [PubMed] [Google Scholar]
- 3.Kearon C, Julian JA, Kovacs MJ, et al. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood. 2008;112:4432–4436. doi: 10.1182/blood-2008-06-163279. [DOI] [PubMed] [Google Scholar]
- 4.Simioni P, Tormene D, Spiezia L, et al. Inherited thrombophilia and venous thromboembolism. Semin Thromb Hemost. 2006;32:700–708. doi: 10.1055/s-2006-951298. [DOI] [PubMed] [Google Scholar]
- 5.De SV, Rossi E, Za T, Leone G. Prophylaxis and treatment of venous thromboembolism in individuals with inherited thrombophilia. Semin Thromb Hemost. 2006;32:767–780. doi: 10.1055/s-2006-955459. [DOI] [PubMed] [Google Scholar]
- 6.De SV, Rossi E, Za T, Leone G. Prophylaxis and treatment of venous thromboembolism in individuals with inherited thrombophilia. Semin Thromb Hemost. 2006;32:767–780. doi: 10.1055/s-2006-955459. [DOI] [PubMed] [Google Scholar]
- 7.Simioni P, Tormene D, Spiezia L, et al. Inherited thrombophilia and venous thromboembolism. Semin Thromb Hemost. 2006;32:700–708. doi: 10.1055/s-2006-951298. [DOI] [PubMed] [Google Scholar]
- 8.Walker PA, Bauer KA, McDonagh J. A simple, automated functional assay for protein C. Am J Clin Pathol. 1989;92:210–213. doi: 10.1093/ajcp/92.2.210. [DOI] [PubMed] [Google Scholar]
- 9.Martinoli JL, Stocker K. Fast functional protein C assay using Protac, a novel protein C activator. Thromb Res. 1986;43:253–264. doi: 10.1016/0049-3848(86)90145-3. [DOI] [PubMed] [Google Scholar]
- 10.Gempeler-Messina PM, Volz K, Buhler B, Muller C. Protein C activators from snake venoms and their diagnostic use. Haemostasis. 2001;31:266–272. doi: 10.1159/000048072. [DOI] [PubMed] [Google Scholar]
- 11.Esmon CT. The endothelial cell protein C receptor. Thromb Haemost. 2000;83:639–643. [PubMed] [Google Scholar]
- 12.Esmon CT. Protein-C: biochemistry, physiology, and clinical implications. Blood. 1983;62:1155–1158. [PubMed] [Google Scholar]
- 13.Esmon CT. The protein C pathway. Chest. 2003;124:26S–32S. doi: 10.1378/chest.124.3_suppl.26s. [DOI] [PubMed] [Google Scholar]
- 14.Oganesyan V, Oganesyan N, Terzyan S, et al. The crystal structure of the endothelial protein C receptor and a bound phospholipid. J Biol Chem. 2002;277:24851–24854. doi: 10.1074/jbc.C200163200. [DOI] [PubMed] [Google Scholar]
- 15.Liaw PC, Mather T, Oganesyan N, Ferrell GL, Esmon CT. Identification of the protein C/activated protein C binding sites on the endothelial cell protein C receptor. Implications for a novel mode of ligand recognition by a major histocompatibility complex class 1- type receptor. J Biol Chem. 2001;276:8364–8370. doi: 10.1074/jbc.M010572200. [DOI] [PubMed] [Google Scholar]
- 16.Kearon C, Ginsberg JS, Kovacs MJ, et al. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med. 2003;349:631–639. doi: 10.1056/NEJMoa035422. [DOI] [PubMed] [Google Scholar]
- 17.Saposnik B, Reny JL, Gaussem P, et al. A haplotype of the EPCR gene is associated with increased plasma levels of sEPCR and is a candidate risk factor for thrombosis. Blood. 2004;103:1311–1318. doi: 10.1182/blood-2003-07-2520. [DOI] [PubMed] [Google Scholar]
- 18.Qu D, Wang Y, Song Y, Esmon NL, Esmon CT. The Ser219-->Gly dimorphism of the endothelial protein C receptor contributes to the higher soluble protein levels observed in individuals with the A3 haplotype. J Thromb Haemost. 2006;4:229–235. doi: 10.1111/j.1538-7836.2005.01676.x. [DOI] [PubMed] [Google Scholar]
- 19.den Dunnen JT, Antonarakis SE. Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum Mutat. 2000;15:7–12. doi: 10.1002/(SICI)1098-1004(200001)15:1<7::AID-HUMU4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 20.Miletich JP, Broze GJ., Jr Beta protein C is not glycosylated at asparagine 329. The rate of translation may influence the frequency of usage at asparagine-X-cysteine sites. J Biol Chem. 1990;265:11397–11404. [PubMed] [Google Scholar]
- 21.Fukudome K, Esmon CT. Identification, cloning, and regulation of a novel endothelial cell protein C/activated protein C receptor. J Biol Chem. 1994;269:26486–26491. [PubMed] [Google Scholar]
- 22.Biguzzi E, Merati G, Liaw PC, et al. A 23bp insertion in the endothelial protein C receptor (EPCR) gene impairs EPCR function. Thromb Haemost. 2001;86:945–948. [PubMed] [Google Scholar]
- 23.Grinnell BW, Walls JD, Gerlitz B. Glycosylation of human protein C affects its secretion, processing, functional activities, and activation by thrombin. J Biol Chem. 1991;266:9778–9785. [PubMed] [Google Scholar]
- 24.Simioni P, Kalafatis M, Millar DS, et al. Compound heterozygous protein C deficiency resulting in the presence of only the beta-form of protein C in plasma. Blood. 1996;88:2101–2108. [PubMed] [Google Scholar]
- 25.Wojcik EG, Simioni P, Berg M, Girolami A, Bertina RM. Mutations which introduce free cysteine residues in the Gla-domain of vitamin K dependent proteins result in the formation of complexes with alpha 1-microglobulin. Thromb Haemost. 1996;75:70–75. [PubMed] [Google Scholar]
- 26.Preston RJ, Villegas-Mendez A, Sun YH, et al. Selective modulation of protein C affinity for EPCR and phospholipids by Gla domain mutation. FEBS J. 2005;272:97–108. doi: 10.1111/j.1432-1033.2004.04401.x. [DOI] [PubMed] [Google Scholar]
- 27.Stearns-Kurosawa DJ, Kurosawa S, Mollica JS, Ferrell GL, Esmon CT. The endothelial cell protein C receptor augments protein C activation by the thrombin-thrombomodulin complex. Proc Natl Acad Sci U S A. 1996;93:10212–10216. doi: 10.1073/pnas.93.19.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fukudome K, Ye X, Tsuneyoshi N, et al. Activation mechanism of anticoagulant protein C in large blood vessels involving the endothelial cell protein C receptor. J Exp Med. 1998;187:1029–1035. doi: 10.1084/jem.187.7.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu JM, Crawley JT, Ferrell G, et al. Disruption of the endothelial cell protein C receptor gene in mice causes placental thrombosis and early embryonic lethality. J Biol Chem. 2002;277:43335–43343. doi: 10.1074/jbc.M207538200. [DOI] [PubMed] [Google Scholar]
- 30.von Depka M, Czwalinna A, Eisert R, et al. Prevalence of a 23bp insertion in exon 3 of the endothelial cell protein C receptor gene in venous thrombophilia. Thromb Haemost. 2001;86:1360–1362. [PubMed] [Google Scholar]
- 31.Akar N, Gokdemir R, Ozel D, Akar E. Endothelial cell protein C receptor (EPCR) gene exon III, 23 bp insertion mutation in the Turkish pediatric thrombotic patients. Thromb Haemost. 2002;88:1068–1069. [PubMed] [Google Scholar]
- 32.Poort SR, Vos HL, Rosendaal FR, Bertina RM. The endothelial protein C receptor (EPCR) 23 bp insert mutation and the risk of venous thrombosis. Thromb Haemost. 2002;88:160–162. [PubMed] [Google Scholar]
- 33.Galligan L, Livingstone W, Mynett-Johnston L, Smith OP. Prevalence of the 23bp endothelial protein C receptor (EPCR) gene insertion in the Irish population. Thromb Haemost. 2002;87:773–774. [PubMed] [Google Scholar]
- 34.Stearns-Kurosawa DJ, Burgin C, Parker D, Comp P, Kurosawa S. Bimodal distribution of soluble endothelial protein C receptor levels in healthy populations. J Thromb Haemost. 2003;1:855–856. doi: 10.1046/j.1538-7836.2003.t01-4-00115.x. [DOI] [PubMed] [Google Scholar]
- 35.Stearns-Kurosawa DJ, Swindle K, D’Angelo A, et al. Plasma levels of endothelial protein C receptor respond to anticoagulant treatment. Blood. 2002;99:526–530. doi: 10.1182/blood.v99.2.526. [DOI] [PubMed] [Google Scholar]
- 36.Qu D, Wang Y, Esmon NL, Esmon CT. Regulated endothelial protein C receptor shedding is mediated by tumor necrosis factor-alpha converting enzyme/ADAM17. J Thromb Haemost. 2007;5:395–402. doi: 10.1111/j.1538-7836.2007.02347.x. [DOI] [PubMed] [Google Scholar]
- 37.Tregouet DA, Heath S, Saut N, et al. Common susceptibility alleles are unlikely to contribute as strongly as the FV and ABO loci to VTE risk: results from a GWAS approach. Blood. 2009;113:5298–5303. doi: 10.1182/blood-2008-11-190389. [DOI] [PubMed] [Google Scholar]
- 38.Heit JA, Armasu SM, Asmann YW, et al. A genome-wide association study of venous thromboembolism identifies risk variants in chromosomes 1q24.2 and 9q. J Thromb Haemost. 2012;10:1521–1531. doi: 10.1111/j.1538-7836.2012.04810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang W, Teichert M, Chasman DI, et al. A genome-wide association study for venous thromboembolism: the extended cohorts for heart and aging research in genomic epidemiology (CHARGE) consortium. Genet Epidemiol. 2013;37:512–521. doi: 10.1002/gepi.21731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gorlov IP, Gorlova OY, Sunyaev SR, Spitz MR, Amos CI. Shifting paradigm of association studies: value of rare single-nucleotide polymorphisms. Am J Hum Genet. 2008;82:100–112. doi: 10.1016/j.ajhg.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schork NJ, Murray SS, Frazer KA, Topol EJ. Common vs. rare allele hypotheses for complex diseases. Curr Opin Genet Dev. 2009;19:212–219. doi: 10.1016/j.gde.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.