Abstract
Anemia is a feature of CKD and a complication of renal transplantation, often caused by impaired production of erythropoietin. The kidney is a target organ for human cytomegalovirus (hCMV) in such patients, but it is not known whether hCMV effects erythropoietin production. We found that kidneys from patients with CKD were positive for hCMV protein and that blood levels of hCMV IgG inversely correlated with red blood cell count. In mice, systemic murine cytomegalovirus infection decreased serum erythropoietin levels. In human erythropoietin-producing cells, hCMV inhibited hypoxia-induced expression of erythropoietin mRNA and protein. hCMV early gene expression was responsible, as ultraviolet-inactivated virus had no effect and valganciclovir treatment showed that late gene expression was nonessential. Hypoxia-induced gene transcription is controlled by the transcription factors hypoxia-inducible transcription factor (HIF)-1α and HIF2α, which are constitutively produced but stable only under low oxygen conditions. We found that hCMV inhibited constitutive production of HIF2α mRNA. HIF2α is thought to be the master regulator of erythropoietin transcription. Single-cell analysis revealed that nuclear accumulation of HIF2α was inhibited in hCMV-infected cells, and the extent of inhibition correlated with hCMV protein expression. Our findings suggest that renal hCMV infection could induce or exacerbate anemia in patients.
Anemia is a frequent feature of CKD and a common complication of renal transplantation. Large cohort studies have shown that around 40% of patients develop anemia following transplant surgery.1–4 Interestingly, the incidence of anemia is unexpectedly high in patients with an otherwise functioning renal transplant (for review, see Vanrenterghem5). Most evidence to date suggests that impaired erythropoietin (EPO) production by the renal allograft is the most common cause of post-transplant anemia.5 In patients with CKD, as renal function decreases the incidence of anemia increases, to >70% in patients with CKD stage 5. Although its cause is multifactorial, a significant factor is inadequate production of EPO. The mechanisms behind the impaired renal EPO production remain to be elucidated.6
EPO is a glycoprotein hormone, produced primarily in the kidney, that controls erythropoiesis and regulates hematocrit.7 Treatment with recombinant EPO can correct post-transplant and renal disease–associated anemia,8 although the cause of EPO loss is unknown. In adults, EPO originates mainly from fibroblast-type cells in the renal cortex9 and is controlled by heterodimeric (α/β) hypoxia-inducible transcription (HIF) factors. Under normoxic conditions, HIFα subunits (HIF1α and HIF2α) are constitutively produced but are subject to rapid proteasomal degradation by the von Hippel-Lindau tumor suppressor, the recognition complex of an E3 ubiquitin ligase. Proteasomal degradation by the von Hippel-Lindau tumor suppressor targeting of HIFα subunits requires the hydroxylation of specific HIFα proline residues by prolyl-4-hydroxylase domain proteins, which require oxygen to function. HIFα proteins are thus spared from degradation under hypoxic conditions, allowing them to bind to the constitutively expressed β-subunit, translocate to the nucleus, and initiate the transcription of hypoxic regulated genes (for review, see Webb et al.10). Recent studies provide convincing evidence that the HIF2α isoform is primarily responsible for the regulation of EPO in the kidney.11,12
Human cytomegalovirus (hCMV) infection is a well recognized risk factor that significantly increases morbidity and mortality rates among renal transplant recipients and patients with CKD.13 The kidney is a target organ for hCMV, and human kidney cells of glomerular, vascular, and tubular origin are susceptible to infection in vitro.14 hCMV DNA and proteins can be detected in renal allografts,15–18 and infection has been associated with severe anemia 6 months after renal transplantation.19 To our knowledge, no studies have detailed its presence or absence in CKD kidneys. However, patients with CKD are frequently hCMV IgM seropositive,20 and immunologic evidence suggests that hCMV is reactivated in this patient population.21 In addition, hCMV-seropositive patients with CKD have lower hemoglobin levels than seronegative patients22 and require a higher dose of erythropoiesis-stimulating agent than do seronegative patients to maintain adequate hemoglobin levels.20,22 Despite the possible association between hCMV infection and the development of anemia, no studies have examined the effect of hCMV infection on cellular EPO production, which could give mechanistic insights into the clinical presentations.
Results
CKD Biopsy Samples Are Positive for hCMV Proteins, and Degree of Anemia Correlates with hCMV IgG Levels
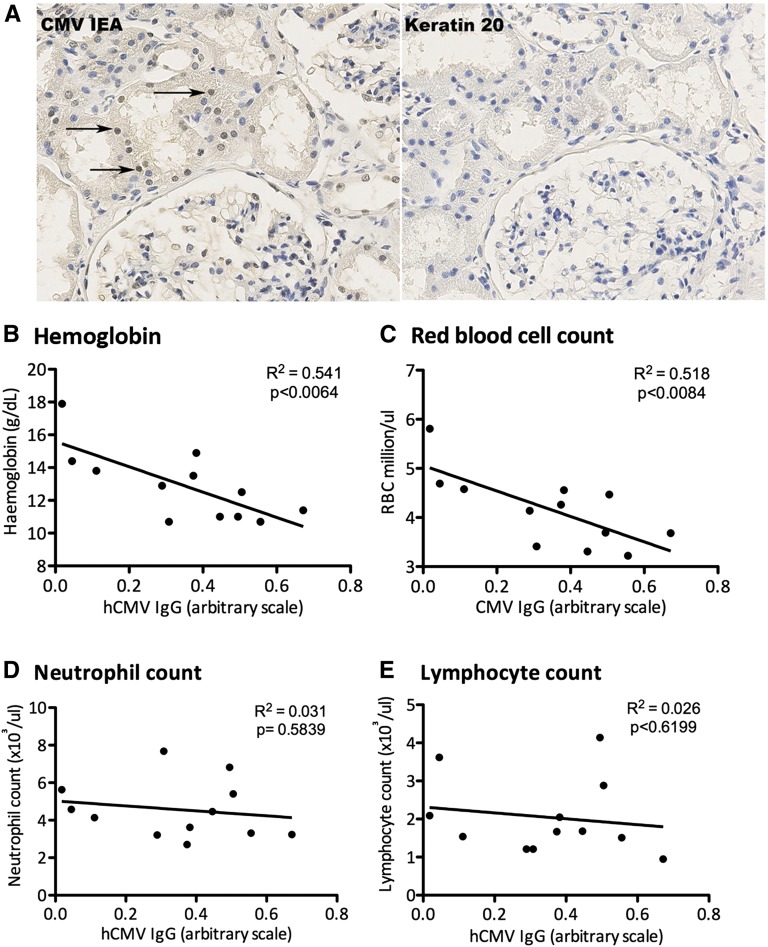
Kidney tissue biopsy specimens or blood samples were collected from patients with CKD (stage 4 renal failure, measured by GFR). In 9 of 13 patients (69%) the kidneys were positive for hCMV immediate early (IE) proteins (Figure 1A). Two of the patients were confirmed to be hCMV IgG seronegative, and biopsy specimens from these patients were negative for hCMV IE staining. One of the highly seropositive IgG patients was positive for low levels of hCMV IgM, but none of the patients had clinical symptoms of active CMV infection. We previously found hCMV protein in allografts from patients with chronic renal allograft nephropathy at the time of nephrectomy.15 To determine whether hCMV antibody titers were linked to anemia, blood samples were analyzed for hCMV IgG by ELISA and for hematologic measures. hCMV IgG inversely correlated with hematocrit (Figure 1B) and red blood cell count (Figure 1C). However, neutrophil (Figure 1D) and lymphocyte (Figure 1E) counts were unaffected. One patient in the cohort was being treated with recombinant EPO, and so they were excluded from this analysis.
Figure 1.
Kidney tissue biopsy specimens from patients with CKD are positive for hCMV, and blood hCMV IgG levels inversely correlate with red blood cell count. (A) Biopsy specimens from CKD were stained for hCMV IE proteins; arrows highlight examples of positive nuclear staining. Images were captured using a magnification factor of ×40. (B–E) Correlation between hCMV IgG levels and serum hemoglobin (B), red blood cell count (C), neutrophil count (D), and lymphocyte count (E).
Infection with Murine CMV Reduces Baseline in Mice
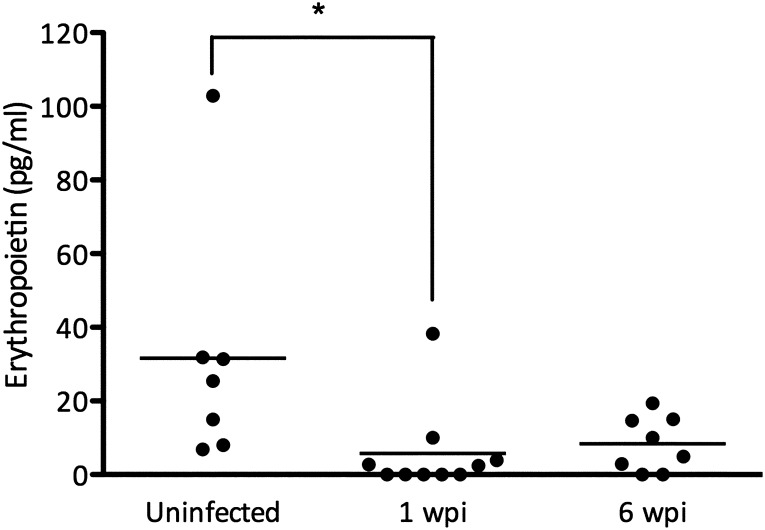
We went on to determine whether CMV infection in mice could decrease circulating EPO (Figure 2). We infected BALB/c mice with murine CMV and euthanized them 1 or 6 weeks after infection. Mean EPO levels were lower in infected mice (mean±SD, 5.7±11.9 pg/ml and 8.4±7.5 pg/ml) than in uninfected controls (31.6±33.1 pg/ml). Hemoglobin and red blood cell counts were not significantly affected, but the mean corpuscular hemoglobin concentration decreased significantly in infected mice (Supplemental Table 1).
Figure 2.
Murine CMV reduces baseline EPO levels in mice. BALB/c mice were left untreated or systemically infected with mCMV by intraperitoneal injection. Circulating EPO levels were determined 1 or 6 weeks after infection (*P<0.05, one-way ANOVA).
hCMV Infects Human EPO-Producing Cells, and Ultraviolet Inactivation Prevents hCMV Protein Expression
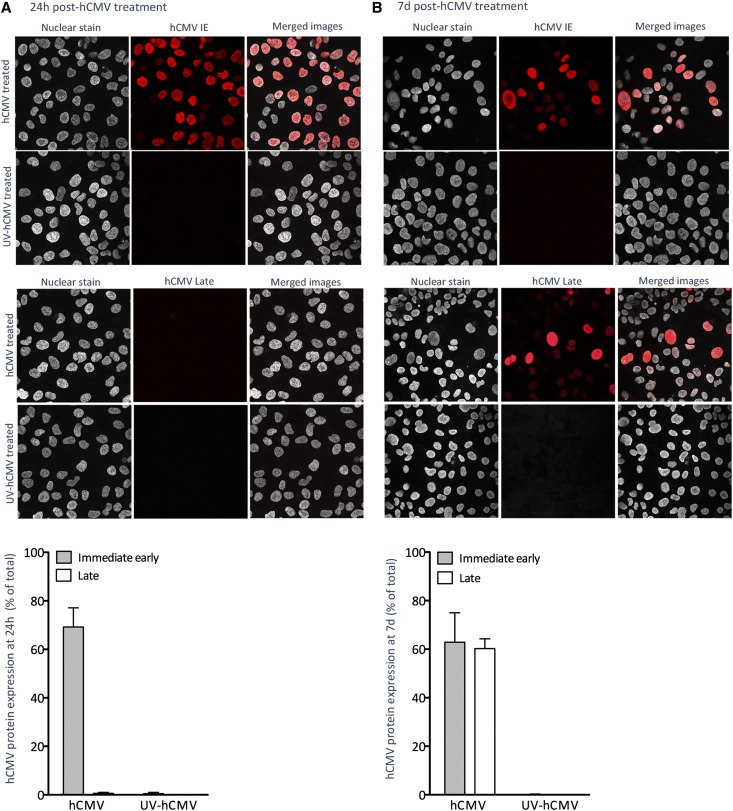
We investigated whether hCMV affected cellular EPO production. We inoculated human EPO-producing cells (HEPCs) with hCMV clinical strain VR1814 at a multiplicity of infection (MOI) of 1, 5, or 10 and cultured them for 24 hours or 7 days. HEPCs treated with hCMV at MOI 5 had the highest level of viable IE-positive cells at both time points (data not shown), so this MOI was used throughout the study, unless otherwise stated.
Approximately 70% of HEPCs were hCMV IE positive by 24 hours after inoculation (Figure 3A), but no late proteins were detected (Figure 3A). At 7 days after inoculation, approximately 60% of HEPCs were positive for both hCMV IE and late proteins (Figure 3B), but the intensity of hCMV IE staining was generally reduced compared with that seen at 24 hours (Figure 3, A and B). In HEPCs inoculated with ultraviolet-inactivated hCMV, no IE or late proteins could be detected at either time point (Figure 3). Thus, HEPCs were susceptible to infection, and late proteins were not expressed until more than 24 hours after inoculation.
Figure 3.
HEPCs are susceptible to hCMV infection, and ultraviolet (UV) inactivation efficiently prevents viral protein expression. Cells were treated with hCMV or ultraviolet-inactivated hCMV at an MOI of 5. Expression of hCMV IE or late antigen was determined by immunocytochemistry and confocal microscopy at 24 hours (A) or 7 days (B) after treatment. Images are representative of three independent experiments. Images were captured using a magnification factor of ×40. Data are mean±SEM from three to eight independent experiments.
hCMV Inhibits Hypoxia-Induced EPO Production
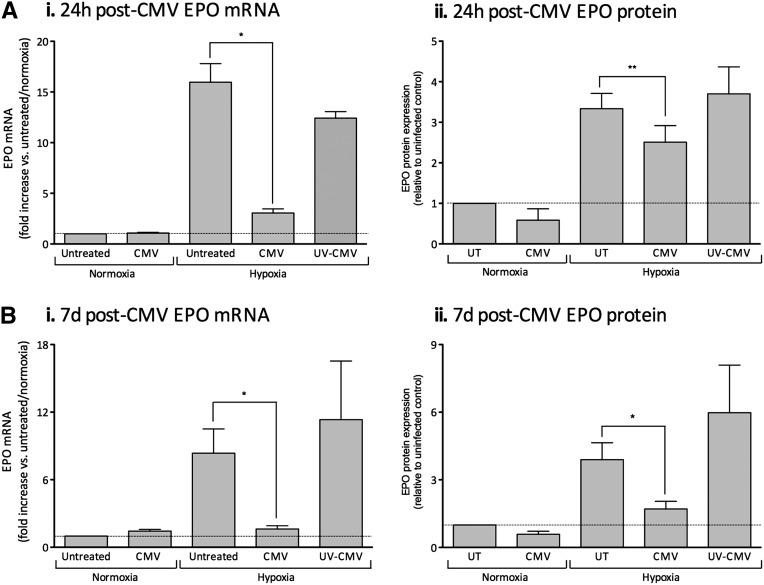
To determine whether hCMV modulated EPO production, we cultured untreated HEPCs, or HEPCs treated with hCMV or ultraviolet-inactivated hCMV, for 24 hours or 7 days before exposure to conditions of normoxia (21% O2) or hypoxia (1% O2) for 20 hours. Under normoxic conditions, HEPCs produced low relative levels of EPO mRNA (mean cycle threshold (Ct)±SEM, 38.4±0.19; mean ΔCt±SEM (18S), 28.79±0.32; n=8), and this constitutive production was not modulated by hCMV infection at 24 hours (Figure 4Ai) or 7 days (Figure 4Bi). HEPC EPO protein production (mean±SEM, 5.99±3.14 mU/ml; n=6) was not significantly modulated by hCMV infection at 24 hours (Figure 4Aii) or 7 days (Figure 4Bii), as determined by ELISA. As expected, we observed an increase in HEPC EPO mRNA and protein levels following exposure to hypoxic conditions for 20 hours (Figure 4). Interestingly, we found that these increases were significantly inhibited when cells were inoculated with hCMV for 24 hours (Figure 4, Ai and Aii) or 7 days (Figure 4, Bi and Bii) before exposure to hypoxia. When hCMV was ultraviolet inactivated before inoculation, hypoxia-induced EPO mRNA and protein expression were not significantly different from the uninfected control (Figure 4), revealing that hCMV gene expression was required.
Figure 4.
hCMV inhibits HEPC EPO production under conditions of hypoxia. Cells were treated with hCMV or ultraviolet (UV)-inactivated hCMV at an MOI of 5 and cultured for 24 hours (A) or 7 days (B) before exposure to 1% O2 or maintenance at normoxia for 18–20 hours before analysis of EPO mRNA production (i) or EPO supernatant protein level (ii) (both plotted relative to the untreated control under normoxic conditions). Data are mean±SEM from three to seven independent experiments. *P<0.05; **P< 0.01, versus untreated (UT).
To determine whether this inhibition was specific to the EPO gene, rather than a general hCMV-induced downregulation of mRNA transcription we measured the expression of IL-6 mRNA, a cytokine known to be upregulated by hCMV infection.23,24 We found that IL-6 mRNA expression was upregulated in hCMV-inoculated HEPCs, under all conditions (mean fold increase±SEM versus untreated controls, 204.6±118.2; n=6; P<0.05 by paired t test). Importantly, these data show that hCMV did not induce a general downregulation of gene transcription.
Next, we confirmed our findings in the EPO-producing cell line HepG2. We found that HepG2 cells were susceptible to infection by hCMV; 70%–80% of the cells expressed hCMV IE protein by 24 hours after inoculation (Supplemental Figure 1A). Under normoxic conditions HepG2 cells expressed higher relative levels of EPO mRNA than HEPC (mean HepG2 Ct±SEM, 32.67±1.9; mean ΔCt±SEM [18S], 20.2±0.8; n=4), and this level increased following hypoxic exposure (Supplemental Figure 1B). hCMV infection inhibited the expression of EPO mRNA (Supplemental Figure 1B), consistent with our observations using HEPC.
To determine whether hCMV-mediated inhibition of hypoxia-induced EPO mRNA and protein expression was a general effect of viral infection, we infected HEPCs with Kaposi's sarcoma-associated herpes virus (KSHV). HEPCs were highly susceptible to infection with rKSHV.219 (Supplemental Figure 2A). We found that levels of EPO mRNA were not modulated by KSHV under normoxic conditions (Supplemental Figure 2B), but under hypoxic conditions EPO mRNA levels were at least 6-fold higher in KSHV-infected HEPCs versus uninfected controls in each experiment. When we ultraviolet-inactivated KSHV before inoculation, we found that EPO mRNA expression was unaffected (Supplemental Figure 2B).
Because we observed inhibition of hypoxia-induced EPO production on hCMV-treated HEPCs as soon as 24 hours after inoculation, we hypothesized that an IE or early hCMV gene product was responsible, as late genes were not expressed at this time point (Figure 3A). Indeed, an inhibitor of hCMV late gene expression (valganciclovir) did not affect the hCMV inhibition of hypoxia-induced EPO protein production (data not shown). Thus, IE or early hCMV gene expression was necessary for the inhibition of EPO production. However, overexpression of IE72 or IE86 alone was not sufficient to recapitulate the effect of hCMV (Supplemental Figure 3), suggesting that the expression of multiple hCMV genes in combination was necessary. In subsequent experiments we used HEPCs inoculated with hCMV 24 hours before exposure to normoxic or hypoxic conditions.
hCMV Inhibits Constitutive HIF2α mRNA Expression
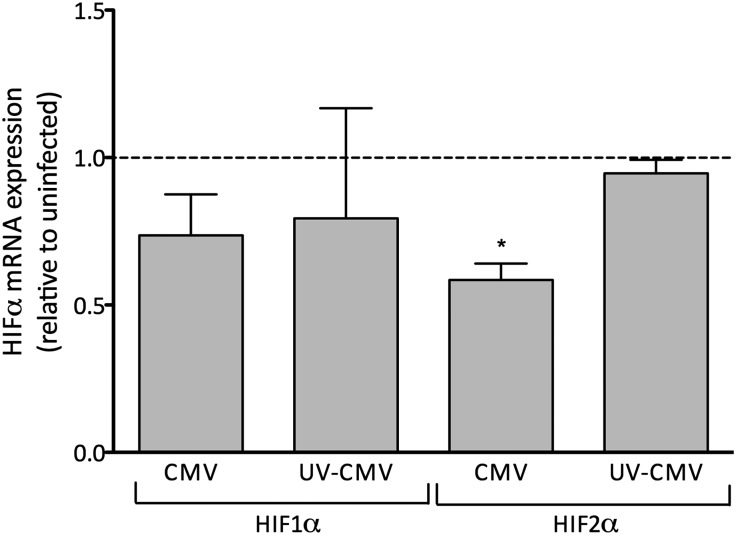
Inhibition of the constitutive transcription of HIFα subunits could explain the inhibition of hypoxia-induced EPO production in hCMV-inoculated HEPCs. Interestingly, we found that constitutive HIF2α transcription was inhibited by hCMV by approximately 40% at 24 hours after inoculation (Figure 5A), whereas the expression of HIF1α was not significantly modified. Because HIF2α, rather than HIF1α, is thought to be the master regulator of EPO gene expression, this finding was consistent with our previous observations.
Figure 5.
hCMV inhibits constitutive HIF2α mRNA expression in HEPCs. Cells were treated with hCMV or ultraviolet (UV)-inactivated hCMV at an MOI of 5 and cultured for 24 hours before exposure to 1% O2 for 18–20 hours. Levels of HIF1α and HIF2α mRNA were determined by qPCR. Data are mean±SEM from three to four independent experiments. *P<0.05, versus untreated.
hCMV Inhibits HIF2α Protein Expression
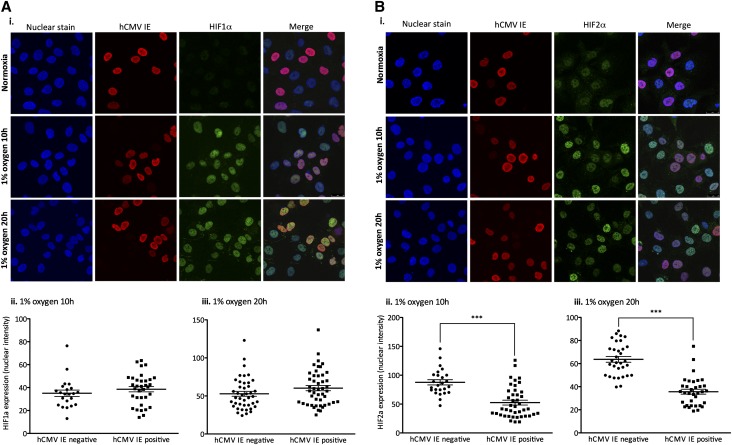
Having discovered that hCMV inhibited constitutive HIF2α mRNA transcription, we sought to determine whether protein accumulation was also affected. We inoculated HEPCs with hCMV at an MOI of 1 to increase the number of uninfected cells in the culture as an internal control for direct comparative analysis. Untreated or hCMV-inoculated HEPCs were exposed to normoxic or hypoxic conditions for 10 or 20 hours, before immunocytochemistry HIF staining. We measured nuclear staining intensity for HIF1α or HIF2α in the nucleus of cells that were categorized as hCMV positive or negative (on the basis of presence or absence of staining for hCMV IE, as a marker of infection). Under normoxic conditions we did not detect HIF1α protein but observed low levels of HIF2α (Figure 6, Ai and Bi). We found that this constitutive HIF2α expression was inhibited in the hCMV-positive population compared with the hCMV-negative population (mean fluorescence intensity±SEM: hCMV negative, 49.69±5.41; hCMV positive, 32.87±0.3.75; n=25 cells/group, two independent sets of experiments; P<0.001). As expected, both HIF1α and HIF2α levels increased following exposure to hypoxia (Figure 6, Ai and Bi). We did not detect any difference in hypoxia-induced nuclear accumulation of HIF1α in HEPCs that were positive or negative for hCMV IE (Figure 6, Ai, Aii, and Aiii). However, under the same conditions we found a significant decrease in nuclear HIF2α in HEPCs that were positive for hCMV IE (Figure 6, Bi, Bii, and Biii) compared with the uninfected cells within the same population. Our results clearly demonstrate that hCMV significantly inhibited nuclear accumulation of HIF2α, but not HIF1α, under conditions of normoxia and hypoxia. These data are consistent with the finding that hCMV inhibited the constitutive expression of HIF2α, but not HIF1α, mRNA.
Figure 6.
hCMV inhibits hypoxia-induced nuclear translocation of HIF2α. HEPCs were treated with hCMV at a MOI of 1 and cultured for 24 hours before exposure to 1% O2, or maintenance at normoxia, for 10 or 20 hours before fixation and staining for HIF1α or HIF2α, and hCMV IE protein expression. Levels of HIF1α or HIF2α, and hCMV IE were observed by confocal microscopy (Ai and Bi). Images were captured using a magnification factor of ×40. Images were analyzed for nuclear fluorescence in the hCMV-negative and -positive populations following exposure to 1% O2 for 10 hours (Aii and Bii) or 20 hours (Aiii and Biii). ***P<0.001.
Hypoxia-Induced HIF2α Protein Levels Are Inversely Proportional to hCMV Protein Expression
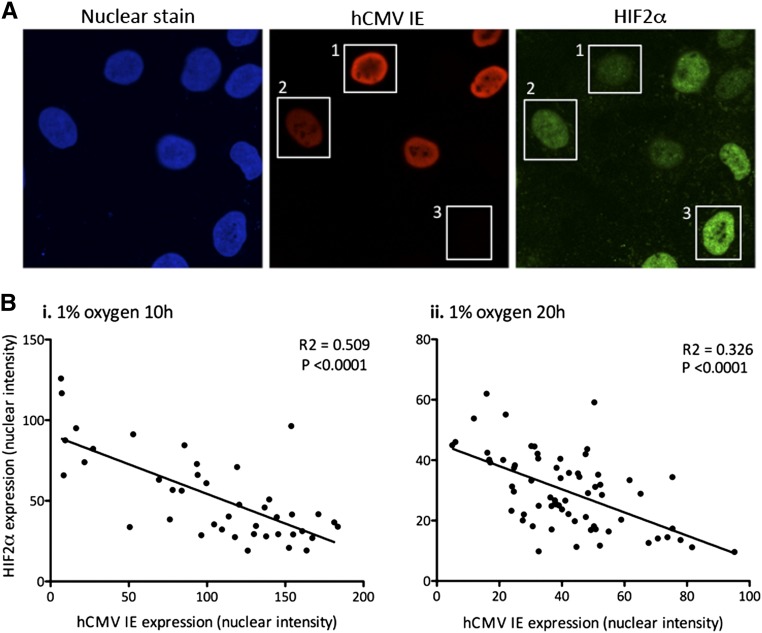
Having determined that hypoxia-induced nuclear accumulation of HIF2α protein was inhibited in hCMV-positive HEPCs, we wished to determine whether there was a quantitative correlation between hCMV infection (measured using IE staining as a general indicator of protein expression) and HIF2α protein. HEPCs that were strongly positive for hCMV IE had very low levels of nuclear HIF2α (Figure 7A, box 1), and those that were more weakly positive had slightly higher levels of HIF2α (Figure 7B, box 2); however, these were still low compared with cells that were negative for hCMV IE (Figure 7A, box 3). We performed quantitative image analysis of staining intensities, which revealed a highly significant negative correlation between hCMV IE expression and nuclear HIF2α, following 10 hours (Figure 7Bi) or 20 hours (Figure 7Bii) of hypoxia. Our results reveal that the level of hCMV gene expression correlated convincingly with the extent of inhibition of HIF2α nuclear expression.
Figure 7.
Expression of hCMV IE negatively correlates with levels of nuclear HIF2α. HEPCs were treated with hCMV at a MOI of 1 and cultured for 24 hours before exposure to 1% O2, or maintenance at normoxia, for either 10 or 20 hours before observation of HIF2α IE protein confocal microscopy. (A) Boxes highlight cells that express (1) high IE protein, low HIF2α; (2) moderate IE protein, moderate HIF2α; and (3) no IE protein, high HIF2α. Images are representative of three independent experiments. Images were captured using a magnification factor of ×63. (B) hCMV-positive cells were analyzed for nuclear intensity of IE protein and HIF2α following exposure to 1% O2 for 10 hours (i) or 20 hours (ii). Data are from three independent experiments.
Discussion
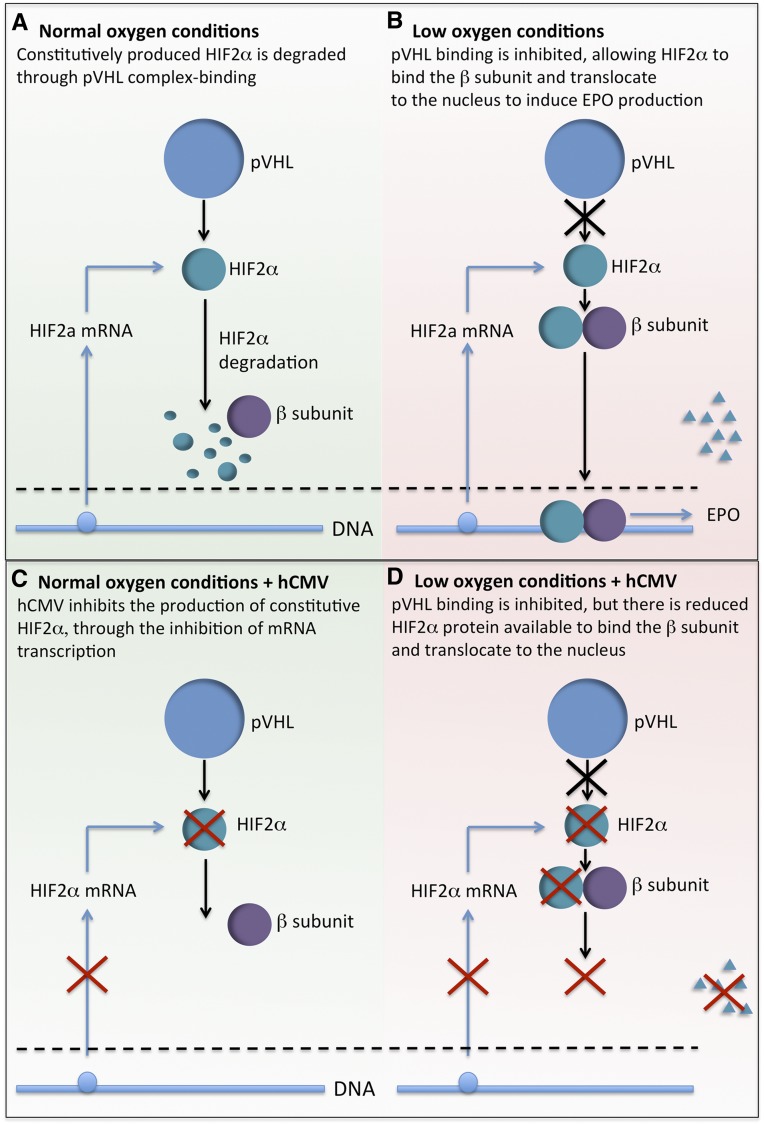
Here we report that hCMV proteins can be detected in CKD kidneys and that hCMV IgG levels correlate with red blood cell counts. In addition, murine CMV infection reduced circulating EPO levels in a mouse model. Importantly, we report that hCMV infection inhibited cellular EPO production. In HEPC, hCMV inhibited hypoxia-induced EPO production as soon as 24 hours after inoculation, but ultraviolet-inactivated virus did not, suggesting that an IE or early hCMV gene was responsible. Indeed, inhibition of late gene expression with valganciclovir did not prevent the inhibition of EPO production. hCMV inhibited constitutive expression of HIF2α, but not HIF1α mRNA. The virus also inhibited hypoxia-induced nuclear accumulation of HIF2α but not HIF1α protein, and the extent of this inhibition closely correlated with the nuclear level of hCMV IE protein. Thus, we propose a working model of hCMV-induced inhibition of HIF2α, and subsequent reduced EPO production under conditions of low oxygen (Figure 8), which could accelerate the development of anemia in patients with renal dysfunction.
Figure 8.
Working model of the effect of hCMV on EPO production. (A) Under conditions of normal oxygen, HIF2α is constitutively produced, bound to proteasomal degradation by the von Hippel-Lindau tumor suppressor (pVHL), and targeted for degradation. (B) Under conditions of low oxygen, pVHL does not bind to HIF2α, so it is spared from degradation. It can then bind to the β-subunit, translocate to the nucleus, and induce the transcription EPO. (C) Under conditions of normal oxygen, hCMV inhibits the constitutive production of HIF2α, but there is no noticeable effect because the protein is usually degraded. (D) Under conditions of low oxygen, the hCMV-induced inhibition of HIF2α protein production results in being less spared from degradation, reduced binding to the β-subunit, and thus reduced transcription of EPO.
Several studies have described the detection of hCMV DNA and proteins in renal allografts.15–18 Previous studies analyzing hCMV IgG titers and hCMV-specific T cells in patients with CKD suggest that hCMV is frequently reactivated in these patients.21,25–27 Our study provides the first direct evidence for the presence of hCMV protein in renal biopsy specimens from CKD kidneys. Furthermore, it is the first to demonstrate an inverse correlation between the level of hCMV IgG and hematocrit in a CKD cohort. Previous studies showed that hCMV-seropositive patients have lower hemoglobin levels than seronegative patients and that they require a higher EPO dose to maintain the goal hemoglobin level,20,22 suggesting that hCMV affects the production of or response to EPO.
In our study, we showed that systemic murine CMV infection of healthy mice resulted in decreased serum EPO levels at 1, but not 6, weeks after infection—possibly because the infection had been controlled by that time. Hemoglobin levels did not significantly decrease, perhaps because the decrease in EPO level was only transient, and erythrocytes have a relatively long life span. We did observe a reduction in the mean corpuscular hemoglobin level that could be an early marker of anemia.
HEPCs were susceptible to hCMV infection, in line with previous work that showed human kidney cells of glomerular, vascular, and tubular origin were also susceptible to infection in vitro.14
Low EPO production is necessary to maintain a normal hematocrit, but EPO gene expression significantly increases under hypoxia. In HEPCs we found that the low constitutive expression of EPO mRNA and protein was substantially increased by exposure to 1% O2, but this increase was significantly inhibited by infection with hCMV before hypoxic exposure. Indeed, this is the first report that hCMV can inhibit the hypoxia response in any cell type. We confirmed this finding using HepG2 cells, which are also susceptible to infection with hCMV.28 Previous studies have shown that HIV-1 infection of the HepG3 cell line resulted in inhibition of hypoxic-induced EPO protein production,29 but this finding most likely reflects a post-translational modification because EPO mRNA levels were not modulated. We found that rKSHV.219 did not inhibit hypoxia-induced EPO production, further evidence that the inhibitory effect of hCMV was not simply a general cellular response to viral infection per se.
EPO is one of a set of hypoxia-inducible genes whose transcription is controlled by heterodimeric (α/β)HIFs.10 Current thinking dictates that such gene expression is regulated by two distinct HIFα isoforms: HIF1α and HIF2α. Under normoxic conditions, HIFα subunits are constitutively produced but are subject to rapid proteasomal degradation, requiring hydroxylation by the oxygen-dependent prolyl-4-hydroxylase domain proteins. They are spared from degradation under conditions of hypoxia, allowing them to bind to the constitutively expressed β-subunit, translocate to the nucleus, and initiate the transcription of hypoxic regulated genes (for review, see Webb et al.10). Here we report that hCMV significantly inhibited the constitutive expression of HIF2α, but not HIF1α, mRNA under normoxic conditions. Correspondingly, the nuclear accumulation of HIF2α was inhibited when HEPCs were exposed to hypoxic conditions, while HIF1α accumulation was unaffected. Following the discovery of the transcription factor HIF1α in the 1990s,30,31 it was widely assumed to be responsible for hypoxia-induced EPO production, and a lively debate ensued about which HIF isoform is responsible for EPO gene expression. Recent studies, in both patients with familial erythrocytosis11,32 and HIF2α conditional knockout murine models,33 provided evidence that in fact HIF2α, not HIF1α, is the master regulator of circulating EPO levels. Perhaps the most convincing evidence that renal HIF2α governs EPO homeostasis came from a study in which murine HIF2α was selectively inactivated by Cre-loxP recombination, to directly determine the contribution of renal HIF2α to EPO homeostasis.12 Upon the loss of HIF2α, the mice developed hypoproliferative anemia and reduced hematocrit, as a consequence of reduced circulating EPO levels.12 Thus, the hCMV-induced inhibition in HIF2α, but not HIF1α activity, could be expected to lead to an inhibition in EPO production.
Our findings suggest that one of the lines of clinical investigation, in the case of a patient with CKD or renal transplant recipient presenting with low blood EPO levels or anemia of unknown cause, should be to determine whether there is active hCMV infection in the kidney. If the biopsy specimen is found to be positive for hCMV proteins, then treatment against persistent viral infection could be considered, in an attempt to correct the anemia though the elimination of possible hCMV-induced inhibition in EPO production. Certainly, further studies are warranted to investigate the effect of such antiviral treatment on the subsequent blood EPO levels in such patients.
Concise Methods
Study Design and Patients
Patients with CKD who had stage 4 renal failure (measured by GFR) were recruited from the Department of General and Vascular Surgery Central Clinical Hospital Ministry of Internal Affairs, Warsaw, Poland. Patients were eligible for arteriovenous fistula construction for hemodialysis, and kidney tissue biopsy specimens or blood samples were collected for this study. The patients were not treated with recombinant EPO unless stated. The patient cohort is further described in Supplemental Table 2. This study was approved by the Ethical Committee of the Central Clinical Hospital Ministry of Internal Affairs (40/2005), and written informed consent was obtained from all patients.
Immunohistochemistry
Paraffin-embedded renal biopsy tissues were cut into sections 4–5 μm thick, dewaxed using xylene (VWR International), and rehydrated in a descending ethanol series (99%–50%). Slides were fixed in 4% neutral buffered formaldehyde (APL) and treated with pepsin (BioGenex) for 3 minutes at 37°C. Antigen retrieval was performed in citrate buffer (BioGenex), and slides were treated with 3% hydrogen peroxidase (Merck), avidin, biotin (DakoCytomation), Fc receptor blocker, and Background Buster Block (Innovex Biosciences), all at room temperature. Mouse monoclonal IgG2a antibodies specific for CMV IE antigen (clone 8B1.2; Chemicon International) or a negative control antibody against keratin 20 (United States Biologic) were added and sections incubated overnight at 4°C. For visualization, we used a horseradish peroxidase detection system (Super Sensitive Link Label Detection System; BioGenex) and positive cells were visualized with chromogen diaminobenzidine (Innovex Biosciences). Slides were counterstained with hematoxylin (Vector Laboratories) and mounted in permanent mounting medium (Histolab Products).
Serological Screening for Detection of HCMV-IgG or IgM
Sera from patients were examined for the presence of hCMV-IgG/IgM using Enzygnost anti-hCMV-IgG/IgM serologic assay kit (Dade Behring, GmbH, Germany) according to manufacturer’s instructions.
Mouse Experiments
BALB/c mice were infected with murine CMV (100,000 plaque forming units dissolved in 0.5–1 ml of PBS) by intraperitoneal injection. The mice were euthanized 1 or 6 weeks after infection, and blood was analyzed for various hematologic measures using an automated hematology analyzer (Sysmex XT 2000i). EPO levels were analyzed with a commercially available ELISA kit (USCN Life Sciences, Inc., Wuhan, China), in accordance with the manufacturer’s instructions.
hCMV Production
The hCMV used was a plaque-purified clinical strain (VR 1814), originally isolated from cervical secretions (a kind gift from Dr. Giuseppe Gerna, University of Pavia, Italy). VR 1814 was propagated in human umbilical vein endothelial cells, and extracellular free virus in the supernatant was titrated by plaque assay to give infectious units/ml. hCMV was inactivated using a Stratagene Stratalinker UV Crosslinker 1800.
Cell Culture
HEPCs or HepG2 were maintained in RPMI (Invitrogen, Paisley, UK) containing 10% FCS and penicillin/streptomycin (all from Sigma-Aldrich). Both were verified mycoplasma-free by MycoAlert mycoplasma detection kit (Clonetics, Lonza). Cultures were dissociated with trypsin/EDTA (Sigma-Aldrich) and passaged into tissue culture multiwell plates (Falcon; Becton Dickinson Labware, Franklin Lakes, NJ), or prefabricated eight-well chamber slides (Lab-Tek II; Thermo Fisher Scientific). Seeding density yielded 90% confluent monolayers for infection within 24 hours.
hCMV and Hypoxia Treatment
hCMV or ultraviolet-inactivated hCMV was diluted in cell culture medium to give an MOI of 1 or 5, before inoculation of HEPCs or HepG2. Untreated cells were handled identically, but without the addition of hCMV or ultraviolet-inactivated hCMV. Cells were incubated for 24 hours before cell culture medium containing hCMV or ultraviolet-inactivated hCMV was removed and replaced. In some experiments, the same protocol was used to infect HEPCs with KSHV (rKSHV.219; gift of Prof. David Blackbourn) at an MOI of 1, which yielded a level of infection at 24 hours similar to that seen with to hCMV used at an MOI of 5. Twenty-four hours or 7 days following the addition of hCMV, ultraviolet-inactivated hCMV or KSHV, HEPC or HepG2 culture was continued under normoxia (atmospheric O2, 5% CO2, 37°C), or hypoxic conditions (1% O2, 5% CO2, 37°C, Invivo 300, Ruskinn) for a further 10–20 hours before sample collection and analysis.
Evaluation of Gene Expression by Quantitative RT-PCR
Total RNA was isolated using an Rneasy mini-kit with QIAshredder (Qiagen) according to the manufacturer’s instruction. RNA was measured with NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific) and then converted into cDNA with Superscripts III First-Strand Synthesis System with Oligo(dT)20 (Invitrogen). cDNA was used for quantitative real-time PCR using TaqMan Fast Universal PCR Master Mix with Applied Biosystems TaqMan MGB primers/probe for EPO, HIF1α, or HIF2α. Either 18S or β2-microglobulin primers were used to quantify expression of housekeeping genes. PCR was performed with the 7900HT Fast Real-Time PCR system (Applied Biosystems), and the results were analyzed with SDS 2.4 software.
Overexpression of hCMV IE72 and IE86 Protein
Plasmids containing IE72 (pGFP-72) or IE86 (pGFP-86) were a kind gift from Professor J.A. Nelson (Oregon Health & Sciences University). Plasmids were transfected into HEPCs using Lipofectamine 2000, according to the manufacturer’s protocol. The transfection medium was replaced 4–6 hours after treatment, and HEPCs were cultured for an additional 24 hours before normoxic/hypoxic exposure and mRNA expression analysis. Successful transfection was confirmed by RT-PCR for IE72 and IE86.
ELISA
Soluble EPO protein in the cell culture supernatant was measured by ELISA, according to the manufacturer’s instructions (EPO Platinum ELISA; eBioscience).
Immunocytochemistry
HEPCs were fixed with formaldehyde, permeabilized with methanol, and blocked with PBS/goat serum (5%). Primary antibodies against hCMV IE, hCMV late antigen (both mouse anti-human; Argene), HIF1a (rabbit anti-human; LifeSpan BioSciences), or HIF2a (rabbit anti-human; Novus Biologicals) were added and incubated overnight at 4°C. Secondary antibodies (Alexa Fluor 647–conjugated goat anti-mouse IgG [H&L; Invitrogen] and/or Alexa Fluor 488–conjugated goat anti-rabbit) were added for 1–2 hours. Cells were mounted with 4′,6-diamidino-2-phenylindole–containing mounting medium (VectaShield) and images were captured by confocal microscopy (Leica) and analyzed with ImageJ software.
Statistical Analyses
Effects of multiple treatments were tested by ANOVA followed by a Bonferroni post hoc test or comparisons of individual treatments by paired or unpaired t test where appropriate. Effects of single treatments were tested by paired t test.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Susanne Schlisio for the use of the hypoxic cell culture chamber, David Blackbourn for providing rKSHV-219, and Lorenz Poellinger for helpiful initial discussions.
This work was supported by the Swedish Medical Research Council K2011-56P-20937-0101-3, Swedish Heart and Lung Foundation (grant no. 20100614, 20100259), Swedish Medical Research Foundation (K2010-56X-12615-13-3, K2012-64X-10857-19-5), Center of Excellence for research on inflammation and cardiovascular disease and the Cardiovascular Program and Stockholm County Council, and Torsten Söderbergs and Ragnar Söderbergs Foundations. It was also supported by the Torsten Söderbergs Foundation, Biltema Foundation, Stichting af Jochnicks Foundation, Sten A Olssons Foundation for Research and Culture, IngaBrit and Arne Lundbergs Foundation, Jane and Dan Olssons Research Foundation, Petrus and Augusta Hedlunds Foundation, Family Erling Perssons Foundation, nxt2b, Swedish Cancer Foundation, Children´s Cancer Foundation, and Stockholm County Council.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Cytomegalovirus and Anemia: Not Just for Transplant Anymore,” on pages 1613–1615.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013101125/-/DCSupplemental.
References
- 1.Lorenz M, Kletzmayr J, Perschl A, Furrer A, Hörl WH, Sunder-Plassmann G: Anemia and iron deficiencies among long-term renal transplant recipients. J Am Soc Nephrol 13: 794–797, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Shah N, Al-Khoury S, Afzali B, Covic A, Roche A, Marsh J, Macdougall IC, Goldsmith DJ: Posttransplantation anemia in adult renal allograft recipients: Prevalence and predictors. Transplantation 81: 1112–1118, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Winkelmayer WC, Chandraker A, Alan Brookhart M, Kramar R, Sunder-Plassmann G: A prospective study of anaemia and long-term outcomes in kidney transplant recipients. Nephrol Dial Transplant 21: 3559–3566, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Vanrenterghem Y, Ponticelli C, Morales JM, Abramowicz D, Baboolal K, Eklund B, Kliem V, Legendre C, Morais Sarmento AL, Vincenti F: Prevalence and management of anemia in renal transplant recipients: A European survey. Am J Transplant 3: 835–845, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Vanrenterghem Y: Anaemia after renal transplantation. Nephrol Dial Transplant 19[Suppl 5]: V54–V58, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Lankhorst CE, Wish JB: Anemia in renal disease: diagnosis and management. Blood Rev 24: 39–47, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Jelkmann W: Molecular biology of erythropoietin. Intern Med 43: 649–659, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Fishbane S: Review: Recombinant human erythropoietin decreases the need for blood transfusions and may delay dialysis in chronic renal failure. ACP J Club 136: 85, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Bachmann S, Le Hir M, Eckardt KU: Co-localization of erythropoietin mRNA and ecto-5′-nucleotidase immunoreactivity in peritubular cells of rat renal cortex indicates that fibroblasts produce erythropoietin. J Histochem Cytochem 41: 335–341, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Webb JD, Coleman ML, Pugh CW: Hypoxia, hypoxia-inducible factors (HIF), HIF hydroxylases and oxygen sensing. Cell Mol Life Sci 66: 3539–3554, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furlow PW, Percy MJ, Sutherland S, Bierl C, McMullin MF, Master SR, Lappin TR, Lee FS: Erythrocytosis-associated HIF-2alpha mutations demonstrate a critical role for residues C-terminal to the hydroxylacceptor proline. J Biol Chem 284: 9050–9058, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapitsinou PP, Liu Q, Unger TL, Rha J, Davidoff O, Keith B, Epstein JA, Moores SL, Erickson-Miller CL, Haase VH: Hepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemia. Blood 116: 3039–3048, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Keyzer K, Van Laecke S, Peeters P, Vanholder R: Human cytomegalovirus and kidney transplantation: A clinician’s update. Am J Kidney Dis 58: 118–126, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Ustinov JA, Loginov RJ, Mattila PM, Nieminen VK, Suni JI, Häyry PJ, Lautenschlager IT: Cytomegalovirus infection of human kidney cells in vitro. Kidney Int 40: 954–960, 1991 [DOI] [PubMed] [Google Scholar]
- 15.Dzabic M, Rahbar A, Yaiw KC, Naghibi M, Religa P, Fellström B, Larsson E, Söderberg-Nauclér C: Intragraft cytomegalovirus protein expression is associated with reduced renal allograft survival. Clin Infect Dis 53: 969–976, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Gerstenkorn C, Robertson H, Mohamed MA, O’Donnell M, Ali S, Talbot D: Detection of cytomegalovirus (CMV) antigens in kidney biopsies and transplant nephrectomies as a marker for renal graft dysfunction. Clin Chem Lab Med 38: 1201–1203, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Li YT, Emery VC, Surah S, Jarmulowicz M, Sweny P, Kidd IM, Griffiths PD, Clark DA: Extensive human cytomegalovirus (HCMV) genomic DNA in the renal tubular epithelium early after renal transplantation: Relationship with HCMV DNAemia and long-term graft function. J Med Virol 82: 85–93, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Helanterä I, Koskinen P, Finne P, Loginov R, Kyllönen L, Salmela K, Grönhagen-Riska C, Lautenschlager I: Persistent cytomegalovirus infection in kidney allografts is associated with inferior graft function and survival. Transpl Int 19: 893–900, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Einollahi B, Lessan-Pezeshki M, Rostami Z, Kalantar E, Afshar R, Beiraghdar F: Anemia after kidney transplantation in adult recipients: Prevalence and risk factors. Transplant Proc 43: 578–580, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Pliquett RU, Klein C, Grünewald T, Ruf BR, Beige J: Lack of evidence for systemic cytomegalovirus reactivation in maintenance hemodialysis patients. Eur J Clin Microbiol Infect Dis 30: 1557–1560, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Yadav AK, Jha V: CD4+CD28null cells are expanded and exhibit a cytolytic profile in end-stage renal disease patients on peritoneal dialysis. Nephrol Dial Transplant 26: 1689–1694, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Betjes MG, Weimar W, Litjens NH: CMV seropositivity determines epoetin dose and hemoglobin levels in patients with CKD. J Am Soc Nephrol 20: 2661–2666, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lagneaux L, Delforge A, Snoeck R, Bosmans E, Moreau JF, Taupin JL, De Clercq E, Stryckmans P, Bron D: Human cytomegalovirus increases constitutive production of interleukin-6 and leukemia inhibitory factor by bone marrow stromal cells. Blood 87: 59–66, 1996 [PubMed] [Google Scholar]
- 24.Geist LJ, Dai LY: Cytomegalovirus modulates interleukin-6 gene expression. Transplantation 62: 653–658, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Betjes MG, Litjens NH, Zietse R: Seropositivity for cytomegalovirus in patients with end-stage renal disease is strongly associated with atherosclerotic disease. Nephrol Dial Transplant 22: 3298–3303, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Wang EC: CD4+ T cells, human cytomegalovirus and end-stage renal disease. Nephrol Dial Transplant 26: 1467–1470, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Betjes MG, Huisman M, Weimar W, Litjens NH: Expansion of cytolytic CD4+CD28- T cells in end-stage renal disease. Kidney Int 74: 760–767, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Derry MC, Sutherland MR, Restall CM, Waisman DM, Pryzdial EL: Annexin 2-mediated enhancement of cytomegalovirus infection opposes inhibition by annexin 1 or annexin 5. J Gen Virol 88: 19–27, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, Goldberg MA, Scadden DT: HIV-1 suppresses erythropoietin production in vitro. Exp Hematol 21: 683–688, 1993 [PubMed] [Google Scholar]
- 30.Wang GL, Jiang BH, Rue EA, Semenza GL: Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A 92: 5510–5514, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Semenza GL, Wang GL: A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 12: 5447–5454, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Percy MJ, Mooney SM, McMullin MF, Flores A, Lappin TR, Lee FS: A common polymorphism in the oxygen-dependent degradation (ODD) domain of hypoxia inducible factor-1alpha (HIF-1alpha) does not impair Pro-564 hydroxylation. Mol Cancer 2: 31, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gruber M, Hu CJ, Johnson RS, Brown EJ, Keith B, Simon MC: Acute postnatal ablation of Hif-2alpha results in anemia. Proc Natl Acad Sci U S A 104: 2301–2306, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.