Abstract
To date, several stem cell labeling protocols have been developed, contributing to a fast growing and promising field of stem cell imaging by MRI (magnetic resonance imaging). Most of these methods utilize iron oxide nanoparticles (MION, SPIO, USPIO, VSIOP) for cell labeling, which provide negative (dark) signal effects on T2-weighted MR images. The following protocol describes stem cell labeling techniques with commercially available gadolinium chelates, which provide positive contrast on T1-weighted MR images, which can be advantageous for specific applications.
Keywords: Magnetic resonance imaging (MRI), Stem cells, Cell labeling, Cell tracking, Cellular imaging, ProHance®, Gadoteridol, Gadolinium chelate
1 Introduction
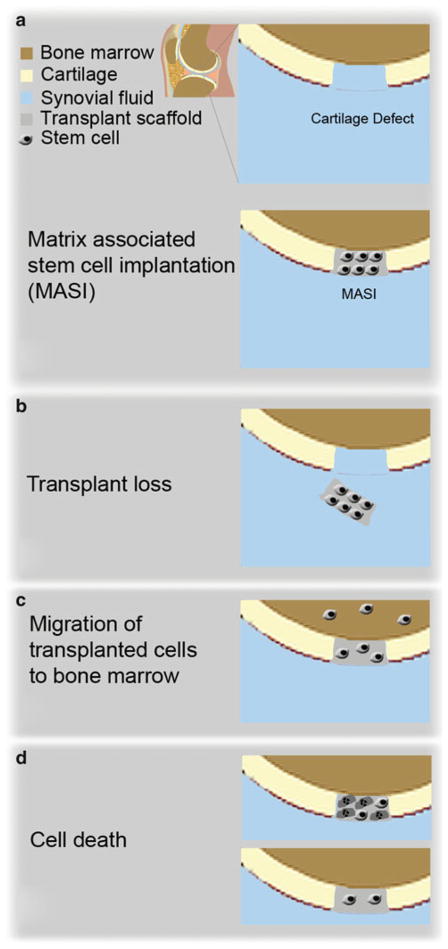
Stem cells represent a unique source for morphological and functional restoration of tissue defects. Preclinical and clinical trials are currently pursued to investigate the potential of various types of stem cells to repair a wide variety of pathological conditions. Autologous adipose-derived stem cells (ADSCs) and bone marrow-derived stem cells (BMSCs) provide several practical benefits including multipotent differentiation capacity, long-term self-renewal, and low immunogenicity (1). However, one of the main limitations for long-term success of stem cell transplants is our inability to recognize the fate of the transplanted cells in a timely manner. To date, a large proportion of transplanted stem cells undergo apoptosis and/or get lost from the transplantation site (2, 3) (Fig. 1). An imaging method that could visualize and track stem cells directly, noninvasively, and repeatedly in vivo could enhance our ability to develop more successful cell transplantation techniques (4, 5). MR imaging is currently the only noninvasive, repeatable diagnostic method, which can provide sub-millimeter anatomical resolution, high soft tissue contrast, and functional information in vivo without radiation exposure (6, 7). Transplanted stem cells can usually not be differentiated from their target organ based on intrinsic signal characteristic. They need to be labeled with contrast agents in order to track them in vivo. Gadolinium-based MR contrast agents have promising properties for MRI cell tracking, including immediate availability for clinical translations and positive (bright) contrast on T1-weighted MR images (8–12).
Fig. 1.
Example of stem cell transplants in a cartilage defect (a) and potential complications, (b) transplant loss, e.g., due to mechanical factors, (c) migration of the implanted cells into bone marrow, and (d) cell apoptosis
This chapter will provide a detailed protocol for effective stem cell labeling with standard small molecular gadolinium chelates and detection of the labeled cells with low field (1-T) and high field (7-T) MR scanners. While we used the nonionic gadolinium-based MRI contrast agent Gadoteridol (ProHance®) for the descriptions below, the same protocol can be applied for cell labeling with Gd-DTPA (Magnevist®), Gadoteric Acid (Dotarem®), Gadodiamine (Omniscan®), or other similar compounds (12–16).
2 Materials
2.1 Equipments
Centrifuge
Microscope
Cell culture incubator (37 °C, 5 % CO2, 90 % humidity)
20/200/1000 μL pipetter and relevant tips
Cell counter and slides or hemocytometer
2.2 Cell Culture
Adipose-derived stem cells (ADSCs) or BMSCs
Sterile conical tube
Tissue culture flask
Dulbecco’s Modified Eagle Medium (DMEM) high glucose (Invitrogen, Carlsbad, CA, catalogue number: 11965) (see Note 1).
Phosphate buffered saline (PBS) 10 mM phosphate, 0.9 % NaCl, pH = 7.4 (Invitrogen, Carlsbad, CA, catalogue number: 14190) (see Note 1).
Trypsin 0.05 %/ethylenediaminetetraacetic acid (EDTA) 0.53 mM solution (see Note 1). Long-term storage of trypsin should be at −80 °C (Mediatech, Manassas, VA, catalogue number: 25200)
Fetal bovine serum (FBS, Invitrogen, Carlsbad, CA, catalogue number: 26140) (see Note 1).
Penicillin/Streptomycin (Invitrogen, Carlsbad, CA, catalogue number: 15140) (see Note 1).
Trypan blue dye (Invitrogen, Carlsbad, CA, catalogue number: 15250)
Collagen Type I solution (Sigma, St. Louis, MO, catalogue number: C2674)
Cell strainer (70 μm, BD biosciences, Bedford, MA, catalogue number: 352350)
2.3 Cell Labeling
ProHance® (Gadoteridol, Gd-HP-DO3A, 0.5 M stock solution) (Bracco Diagnostics, catalogue number: 0270-1111-03)
Lipofectin® reagent (Invitrogen, Carlsbad, CA, catalogue number: 18292-011)
2.4 Gadolinium Quantification
Hydrochloric acid (HCl), metal grade (Fisher Scientific, Fair Lawn, NJ, catalogue number: A508-500)
Filter 0.2 μm pore size (Thermo Scientific Nalgene® syringe filter, catalogue number: 190-2520)
2.5 Magnetic Resonance Imaging
NMR tubes 3 mm (Bruker, catalogue number: Z117723) or PCR tubes (Thermowell™ Tube, Corning Incorporation, NY, catalogue number: 6571)
NMR or PCR tube holder (homemade)
MR scanner (we used ASPECT 1 T MR scanner and Varian 7 T MR scanner with GE interface)
3 Methods
Labeling stem cells with MR contrast agents enables us to visualize the cells in vivo, and ensure that they have been delivered to the target site. Possible applications include evaluations of different numbers of transplanted cells, different time points of transplantations and effects of scaffolds and growth factors on tissue regeneration outcomes. In addition, in vivo cell tracking techniques enable us to detect complications of the engraftment process, such as stem cell loss, apoptosis, or rejection (Fig. 1).
The protocols described here have been optimized for labeling of adipose derive stem cells (ADSCs) and bone marrow-derived mesenchymal stem cells (MSCs) with small molecular gadolinium chelates, and related detection of the labeled stem cells with MR imaging. Labeling of other cell types may require modifications and adjustments of this protocol.
This protocol pertains to (1) stem cell isolation and culturing (2) labeling of stem cells with Gadoteridol, (3) preparation for imaging, (4) Gadolinium quantification, and (5) MR imaging of the labeled cells and post imaging analysis:
3.1 Cell Culturing
-
Isolation and expansion of adipose-derived stem cells (ADSCs).
After harvesting the adipose tissue from the rat, keep the tissue in cold PBS.
Move the adipose tissue from the tubes to a sterile Petri dish.
Add 5–10 mL of PBS to prevent dehydration.
Cut the tissue into small pieces (<2–3 mm3) using a sterile scalpel.
To digest the tissue, transfer the homogenized adipose tissue into a conical tube, add 0.075 % Collagen Type I solution.
Incubate the tube for 60 min at 37 °C with an agitation every 10–20 min.
After 60–90 min of incubation, the adipose tissue will be digested and disappear.
After digestion, centrifuge the tube(s) at room temperature, for 10 min at 500 × g.
Gently, remove the saline supernatant and fat layer from the tube (Be careful not to remove the cell pellet).
Add 10 mL PBS, resuspend the pellet, and filter the cell suspension through a 70 μm cell strainer.
Centrifuge at 500 × g for 5 min at room temperature and throw away the supernatant.
Resuspend the cell pellet in 10 mL of the complete cell culture medium (high glucose DMEM supplemented with 10 % FBS and 100 IU Penicillin and 100 μg/mL Streptomycin)
Seed the cells in 25 cm2 cell culture flask.
After 24 h, change the cell culture medium.
-
Isolation and expansion of Human mesenchymal stem cells (hMSCs).
Human bone marrow-derived mesenchymal stem cells (hMSCs) can be harvested from aspirated bone marrow (from iliac crest and/or sternum) of healthy donors or can be bought from Lonza (catalogue number PT-2501).
Bone marrow aspirates are diluted in DMEM and centrifuged two times for 10 min at 500 × g.
Cell pellet resuspended in complete medium containing high glucose DMEM supplemented with 10 % FBS and 100 IU Penicillin and 100 μg/mL Streptomycin, are plated in a 75 cm2 cell culture flask.
After 48 h, to remove the nonadherent cells, wash the cells with prewarmed PBS and change the PBS with prewarmed fresh medium. The medium is replaced every 2–3 days as the cells grow to confluence (see Note 2).
3.2 Cell Labeling
Cells are labeled with Gadoteridol (ProHance®, see Note 3). In order to facilitate efficient transfection of the contrast agent into cells, the transfection agent Lipofectin® is used (see Notes 4 and 5).
Add 0/1/2/4 μL/mL of Lipofectin® at concentrations of 0/0.5/1/2 mM to four 1 mL tubes containing 500 μL of DMEM each. Allow the solution to sit 15 min for the Lipofectin® to distribute homogenously.
Add 0/1/2/4 μL/mL of Gadoteridol (ProHance®) at concentrations of 0/0.5/1/2 mM to four 1 mL tubes containing 500 μL of Lipofectin® plus DMEM each. Allow the solution to sit for 30 min more for the Lipofectin® to complex with ProHance®.
Prepare the labeling medium by adding 8 mL DMEM (final volume will be 9 mL).
Aspirate the stem cell medium and wash the cells with prewarmed PBS one time to remove any dead cells and the remaining cell medium.
Add the labeling medium to each flask containing the attached stem cells. Incubate the cells with the serum free labeling medium for 4 h.
After 4 h, supplement the labeling medium with 10 % FBS (1 mL) and 100 IU/mL Penicillin and 100 μg/mL Streptomycin (100 μL of 100× pen/strep) in each flask and continue labeling for 24 h (see Note 6).
3.3 Cell Detachment and Preparation for Imaging
Wash the cells in each flask with 5 mL of PBS for each flask three times (see Note 7).
Add 3 mL of Trypsin to each flask and place them in 37 °C incubator for 2–5 min (see Note 8).
Add 3 mL of complete medium to each flask to neutralize Trypsin activity.
Remove the 6 mL of medium from each tube and add them to their respective saved medium and PBS.
Spin the cells down at 500 × g for 5 min. Aspirate the supernatant and resuspend in 1 mL of complete medium for each of the four labeling concentrations.
Mix 10 μL of Trypan blue and 10 μL of the detached cell suspension of each four samples. Gently mix the two solutions and place 10 μL of this solution onto a cell counter slide and count the number of cells per mL of cell suspension.
Take an equal number of cells (e.g., 2 × 106 cells) from each concentration to create a uniform cell pellet for MR imaging. Spin the cells down at 500 × g for 5 min. Resuspend each of the samples in 80μL of complete medium.
Transfer the 80 μL from each sample into an NMR tube for MR imaging. Place the NMR tubes into 1 mL centrifuge tubes and spin them down at 500 × g for 5 min.
3.4 Gadolinium Quantification
Take an equal number of cells (e.g., 1 × 106 cells) from each concentration to prepare the samples for Gadolinium (Gd) measurement by inductively coupled plasma optical emission spectrometry (ICP-OES).
Dissolve the cell pallets with 200 μL of 70 % HCl (metal grade) and incubate overnight at room temperature.
Top up the samples with DI water to 5 mL.
Filter the samples with a 0.2 μm pore size filter.
Prepare your blank (DI water) and low concentration (1 ppm) and high concentration (2 ppm) Gd standards and send the samples and standards for ICP-OES.
3.5 Cell Imaging and T1 Relaxation Time Calculation
Place each NMR tube into a slot in an MR tube holder filled with fluid (e.g., PBS). Put the holder in an MR coil and place the holder and coil in the center of the magnet of the MR scanner.
Scan the cells with a T1-weighted spin echo (T1W SE) or gradient echo (GE) sequence to visualize the gadolinium-labeled cells. Parameters for a T1-weighted spin echo (T1W SE) sequence are 500 ms repetition time (TR); 13–17 ms echo time (TE); 90° flip angle (FA); 256 × 256 image matrix; 0.5–1 mm slice thickness (ST): and 8 number of excitation (NEX) (Fig. 2). These parameters may have to be adjusted, depending on the applied field strength, medium, background signal, number of cells, etc.
To calculate T1 relaxation times of labeled cells, an inversion recovery spin echo (IR-SE) sequence with varying inversion times (TI) can be used. Examples of parameters for our typical IR-SE are repetition time (TR) 8,000 ms; echo time (TE) 8 ms; and TI 4,000, 2,000, 1,000, 500, 250, 100, 50, 25 ms; FA of 90°; image matrix 128 × 128; slice thickness (ST) 0.5–1 mm: and 1–2 number of excitation (NEX).
After completion of the MR scan, images should be imported to a DICOM viewer for post-processing with specific software, such as Osirix software (free download via the Internet).
-
The signal intensity for each cell pellet can be measured via an operator defined region of interest (ROI).
The data can be further analyzed with excel software to calculate T1 relaxation times of the labeled cells, by using the following algorithm:where ln is the logarithm to the base e (natural logarithm), SI is the signal intensity, TI is inversion recovery time, and MaxSI is the absolute maximum signal intensity between the inversion times.
Then, the new value for each inversion recovery is calculated.
- Fit a linear curve to the calculated values, use the slope of the equation as R1 values and calculate the T1 relaxation times by the following formula:
Fig. 2.
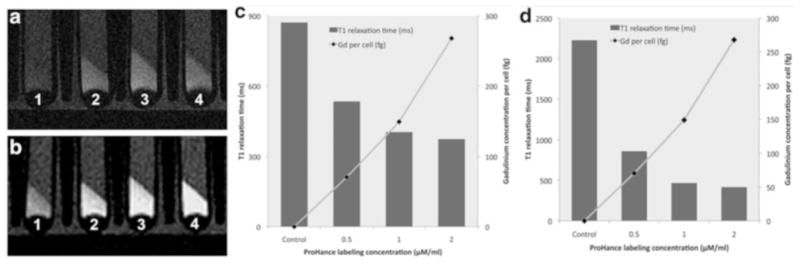
Sagittal MR Imaging of pellets of 2 × 106 unlabeled (left) and labeled ADSCs (0.5, 1, and 2 mM Gadoteridol) in test tubes: (a) T1W SE 500/17 (TR/TE) at 7 T, (b) T1W SE 500/13 (TR/TE) at 1 T; panels (c, d) show corresponding T1 relaxation times of labeled and unlabeled ADSCs, determined based on IR sequences, as well as corresponding Gd content of the labeled cells, as measured by inductively coupled plasma (ICP)
4 Discussion and Conclusion
Figure 2 shows the T1 relaxation times of ADSCs, which were labeled with different concentrations of Gadoteridol and Lipofectin. The ICP-OES analysis of our samples showed no detectable Gd content in unlabeled control ADSCs, and an increasing Gd content in cells incubated with increasing concentrations of Gadoteridol. As expected, increasing Gadoteridol incubation concentrations yielded shorter T1 relaxation times at both 1 T and 7 T magnetic field strengths. In conclusion, we described a method for labeling stem cells with commercially available small molecular gadolinium chelates (ProHance®/Gadoteridol), which provide positive (bright) signal effects on T1-weighted MR images.
Footnotes
DMEM, PBS, and Trypsin should be prewarmed to 37 °C in a water bath before use.
The cells are lifted by incubation with 0.5 % Trypsin. The first passage after plating is usually taken as P0. Cells can be expanded up to passage 6 (P6) though the efficiency of cell labeling decreases with the number of passages.
ProHance® (Gadoteridol), is a gadolinium-based contrast medium that belongs to the MRI class of small molecular paramagnetic contrast agents (ATC Class V08CA). Each milliliter of ProHance® contains 279.3 mg Gadoteridol, 0.23 mg Calteridol calcium, 1.21 mg tromethamine and water. Gadoteridol is the active pharmaceutical ingredient of ProHance®, with a molecular structure of a macrocyclic, nonionic, and high stability chelate. ProHance® contains no antimicrobial preservative and has a pH of 6.5–8.0.
Lipofectin®, a reagent composed of the cationic lipids DOTMA and DOPE in a 1:1 mixture in membrane-filtered water, is used to transfect RNA or DNA. However, Lipofectin® transfection has also been used in labeling with contrast agent (17, 18).
Negatively charged MR contrast agents likely complex with positively charged lipid molecules. After fusing to the cell membrane these complexes release the MR contrast agents into the cytosol (19, 20).
Alternatively, the cells may be labeled for 48 h instead of 24.
Under a microscope, observe the cells to determine that they do not detach from the flask. If cells are detached, stop washing or use the complete prewarmed medium instead of PBS.
Before proceeding to the next step, check the cells under the microscope to determine if they are detached from the flask. If they are still attached, place the flasks in an incubator for another 2 min to allow the Trypsin to cleave the attachments. Repeat until more than 70–80 % of the cells are detached.
References
- 1.Yuan L, Sakamoto N, Song G, Sato M. Migration of human mesenchymal stem cells under low shear stress mediated by MAPK signaling. Stem Cells Dev. 2012;21(13):2520–2530. doi: 10.1089/scd.2012.0010. [DOI] [PubMed] [Google Scholar]
- 2.Berman SM, Walczak P, Bulte JW. MRI of transplanted neural stem cells. Methods Mol Biol. 2011;711:435–449. doi: 10.1007/978-1-61737-992-5_22. [DOI] [PubMed] [Google Scholar]
- 3.Biancone L, Crich SG, Cantaluppi V, et al. Magnetic resonance imaging of gadolinium-labeled pancreatic islets for experimental transplantation. NMR Biomed. 2007;20:40–48. doi: 10.1002/nbm.1088. [DOI] [PubMed] [Google Scholar]
- 4.Modo M, Meade TJ, Mitry RR. Liver cell labelling with MRI contrast agents. Methods Mol Biol. 2009;481:207–219. doi: 10.1007/978-1-59745-201-4_17. [DOI] [PubMed] [Google Scholar]
- 5.Sykova E, Jendelova P, Herynek V. Magnetic resonance imaging of stem cell migration. Methods Mol Biol. 2011;750:79–90. doi: 10.1007/978-1-61779-145-1_5. [DOI] [PubMed] [Google Scholar]
- 6.Kedziorek DA, Kraitchman DL. Superparamagnetic iron oxide labeling of stem cells for MRI tracking and delivery in cardiovascular disease. Methods Mol Biol. 2010;660:171–183. doi: 10.1007/978-1-60761-705-1_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hedlund A, Ahren M, Gustafsson H, et al. Gd2O3 nanoparticles in hematopoietic cells for MRI contrast enhancement. Int J Nanomedicine. 2011;6:3233–3240. doi: 10.2147/IJN.S23940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kraitchman DL, Kedziorek DA, Bulte JW. MR imaging of transplanted stem cells in myocardial infarction. Methods Mol Biol. 2011;680:141–152. doi: 10.1007/978-1-60761-901-7_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Major JL, Meade TJ. Bioresponsive, cell-penetrating, and multimeric MR contrast agents. Acc Chem Res. 2009;42:893–903. doi: 10.1021/ar800245h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caravan P. Strategies for increasing the sensitivity of gadolinium based MRI contrast agents. Chem Soc Rev. 2006;35:512–523. doi: 10.1039/b510982p. [DOI] [PubMed] [Google Scholar]
- 11.Villaraza AJ, Bumb A, Brechbiel MW. Macromolecules, dendrimers, and nanomaterials in magnetic resonance imaging: the interplay between size, function, and pharmacokinetics. Chem Rev. 2010;110:2921–2959. doi: 10.1021/cr900232t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tweedle MF. The ProHance story: the making of a novel MRI contrast agent. Eur Radiol. 1997;7(5):225–230. doi: 10.1007/pl00006897. [DOI] [PubMed] [Google Scholar]
- 13.Henning TD, Saborowski O, Golovko D, et al. Cell labeling with the positive MR contrast agent Gadofluorine M. Eur Radiol. 2007;17:1226–1234. doi: 10.1007/s00330-006-0522-9. [DOI] [PubMed] [Google Scholar]
- 14.Henning TD, Wendland MF, Golovko D, et al. Relaxation effects of ferucarbotran-labeled mesenchymal stem cells at 1.5 T and 3 T: discrimination of viable from lysed cells. Magn Reson Med. 2009;62:325–332. doi: 10.1002/mrm.22011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henning TD, Gawande R, Khurana A, et al. MR imaging of ferumoxides labeled mesenchymal stem cells in cartilage defects: in vitro and in vivo investigations. Mol Imaging. 2012;11(3):197–209. [PMC free article] [PubMed] [Google Scholar]
- 16.Nejadnik H, Henning TD, Boddington S, et al. Somatic differentiation and MR imaging of magnetically labeled human embryonic stem cells. Cell Transplant. 2012;21(12):2555–2567. doi: 10.3727/096368912X653156. [DOI] [PubMed] [Google Scholar]
- 17.Rudelius M, Daldrup-Link HE, Heinzmann U, et al. Highly efficient paramagnetic labelling of embryonic and neuronal stem cells. Eur J Nucl Med Mol Imaging. 2003;30:1038–1044. doi: 10.1007/s00259-002-1110-0. [DOI] [PubMed] [Google Scholar]
- 18.Nejadnik H, Henning TD, Thuy D, et al. MR imaging features of gadofluorine-labeled matrix-associated stem cell implants in cartilage defects. PLoS One. 2012;7(12):e49971. doi: 10.1371/journal.pone.0049971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daldrup-Link HE, Rudelius M, Oostendorp RA, et al. Targeting of hematopoietic progenitor cells with MR contrast agents. Radiology. 2003;228:760–767. doi: 10.1148/radiol.2283020322. [DOI] [PubMed] [Google Scholar]
- 20.Felgner PL, Gadek TR, Holm M, et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]