Abstract
We investigated the role of common genetic variation in immune-related genes on breast cancer disease-free survival (DFS) in Korean women. 107 breast cancer patients of the Seoul Breast Cancer Study (SEBCS) were selected for this study. A total of 2,432 tag single nucleotide polymorphisms (SNPs) in 283 immune-related genes were genotyped with the GoldenGate Oligonucleotide pool assay (OPA). A multivariate Cox-proportional hazard model and polygenic risk score model were used to estimate the effects of SNPs on breast cancer prognosis. Harrell’s C index was calculated to estimate the predictive accuracy of polygenic risk score model. Subsequently, an extended gene set enrichment analysis (GSEA-SNP) was conducted to approximate the biological pathway. In addition, to confirm our results with current evidence, previous studies were systematically reviewed. Sixty-two SNPs were statistically significant at p-value less than 0.05. The most significant SNPs were rs1952438 in SOCS4 gene (hazard ratio (HR) = 11.99, 95% CI = 3.62–39.72, P = 4.84E-05), rs2289278 in TSLP gene (HR = 4.25, 95% CI = 2.10–8.62, P = 5.99E-05) and rs2074724 in HGF gene (HR = 4.63, 95% CI = 2.18–9.87, P = 7.04E-05). In the polygenic risk score model, the HR of women in the 3rd tertile was 6.78 (95% CI = 1.48–31.06) compared to patients in the 1st tertile of polygenic risk score. Harrell’s C index was 0.813 with total patients and 0.924 in 4-fold cross validation. In the pathway analysis, 18 pathways were significantly associated with breast cancer prognosis (P<0.1). The IL-6R, IL-8, IL-10RB, IL-12A, and IL-12B was associated with the prognosis of cancer in data of both our study and a previous study. Therefore, our results suggest that genetic polymorphisms in immune-related genes have relevance to breast cancer prognosis among Korean women.
Introduction
Cancer is a significant health problem in many parts of the worldwide [1], [2]. In Korea, the incidence rate of breast cancer was ranked second and the mortality rate fifth in Korean women, which steadily increased from 1983 to 2010 [3]. The etiology and progression of breast cancer is a multiple-step process caused by combining many factors which involve environmental, hormonal and genetic factors [4], [5]. We focused on genetic factors involved in immune response which was known to play a role in breast cancer prognosis.
The association of immune markers with breast cancer prognosis were well known and the role as key factor of microenvironment of tumor such as tumor suppressor or growth. For example, high density of CD68 which is high-infiltration of tumor-associated macrophages was related with poorer outcome in node-negative breast cancer [6] and CD44 positive patients showed longer overall survival and progression free survival than CD44 negative patients [7]. In addition, cytokines produced by various immune cells were known to modulate the transition from the innate to the adaptive immune response, the activation of anti-tumor cells, persistent oxidative stress, and the angiogenesis of breast cancer [8]–[10]. The prognosis of breast cancer was also known to be associated with single nucleotide polymorphisms (SNPs) in the immune system related genes [11]–[14]. Those reports described that genetic variants of toll-like receptor 4 (TLR4), interleukin 12 (IL-12), interleukin 2 (IL-2), and interleukin 6 (IL-6) were related with breast cancer prognosis. However, there have been few studies that investigate the association between comprehensive list of variants in the immunity-related genes and the prognosis of breast cancer.
Given the findings that immune system is related with breast cancer prognosis, we hypothesized that many genetic polymorphisms in immune related genes might be prognostic factor of breast cancer recurrence. In this study, the role of common immune genetic variations to the disease free survival (DFS) of breast cancer was investigated with the multivariate Cox-proportional hazard model by individual variants, polygenic risk score model, and an extended gene set enrichment analysis. Additionally, a systematic review of previous literature that had reported on the associations between variants of the immunity-related genes and the prognosis of various cancers was done.
Materials and Methods
Study population
Among subjects of Seoul Breast Cancer Study (SEBCS), a multicenter based case-control study recruiting between 2001 and 2007, the participants in this study were patients diagnosed with histologically confirmed breast cancer in the Seoul National University Hospital during 2002–2004. Based on the sample availability and quality of DNA, 140 breast cancer patients were successfully genotyped [15]. Among them, 107 patients were included in the final analysis after excluding patients without survival status or clinical information or been diagnosed as metastatic breast cancer patients.
During recruitment, well-trained interviewers provided patients with informed consent forms and collected information with a structured questionnaire. Through abstracting the medical chart, information on survival status, hormone receptor status, and TNM stage [16] were obtained.
This study design was approved by the Committee on Human Research of Seoul National University Hospital (IRB No. H-0503-144-004).
Genotyping
Among 209 samples met the genotyping criteria (concentration >7.5 ng/ul and total amount of DNA >750 ng), 140 cases were successfully genotyped. 283 immune-related candidate genes were composed of 190 innate immune-related genes in innate immune oligonucleotide pool assay (OPA) chip and 93 adaptive immune-related genes in Non-Hodgkin’s lymphoma (NHL) OPA chip as described in previous study [15], [17]. 2,432 Tags SNPs were selected with SNP500 Cancer project database considering the site from 20 kb upstream of the first site of transcription of a candidate gene to 10 kb downstream of the end site of the last exon of the candidate gene and genotyped. Among them, 461 SNPs were excluded from the analysis because of low minor allele frequency (MAF) (<3%) and deviation from Hardy-Weinberg Equilibrium (HWE) (P<10−4). Finally, a total of 1,971 SNPs in 279 immunity genes were selected for the analysis.
Statistical method
A DFS was calculated from the date when patients underwent a breast cancer operation to the date of last follow-up or recurrence, such as loco-regional, distant, contralateral recurrence and death from any causes. If patients had no evidence of recurrence, they were censored at the last follow-up date or on June 30, 2011. The median follow-up time was 4.87 years (range, 0.25–6.72 years).
Demographic data including age (<50 and ≥50), body mass index (BMI) (<21.4 and ≥21.4), family history of breast cancer in 1st and 2nd relatives (no and yes), educational level (≤ middle school, high school, and ≥ college or university), smoking status (never and ever), alcohol consumption (never and ever), and menopausal status (premenopausal and postmenopausal), and clinicopathological data including estrogen receptor status (ER) (positive and negative), progesterone receptor status (PR) (positive and negative), and 7th AJCC TNM stage (I, II, and III) were assessed for DFS with the log-rank test and univariate Cox-proportional hazard model. Multivariate Cox-proportional hazard model adjusted for age, ER status, PR status, and TNM stage (I, II, and III) was used to calculate the hazard ratio (HR) and their 95% CI of the effect for each SNP on the DFS of breast cancer based on additive genetic models. If SNPs were located in the same candidate gene and these SNPs had a linkage disequilibrium (LD) (r2>0.4), the most significantly associated SNP were selected. To correct the multiple comparison, false discovery rate (FDR) p-values were calculated with the Benjamin-Hochberg method [18].
For the polygenic risk score method, the polygenic risk score was calculated by adding the number of risk alleles in each patient based on individual SNP analyses and the patients were categorized into tertiles of polygenic risk score [19]. HR and 95% confidence intervals (CIs) per tertile of polygenic risk score were calculated. After analyzing multivariate Cox-proportional hazard model, Harrell’s C index was calculated to evaluate predictive accuracy of polygenic risk score model [20]. In addition, 4-fold cross-validation method was used to appraise the internal validity of our model; the entire data set was randomly partitioned into 4 equal size subsets. Of the 4 subsets, 3 subsets were used as training data, and a remaining single subset was retained as the validation data for testing the model. Significantly associated SNPs with prognosis of breast cancer were firstly estimated in training set and then Harrell’s C index was estimated based on those SNPs in validation set. The cross-validation process was then repeated 4 times. The summary of these 4 Harrell’s indices was assessed by fixed-effect model meta-analysis.
The GSEA-SNP method was used to reveal the biological function of the SNPs which were significantly related to breast cancer prognosis [21]. Pathway information was obtained from the Molecular Signatures Database (MSigDB) which collected annotated gene sets from the following online databases; BioCarta, KEGG, Pathway Interaction Database, Reactome, SigmaAldrich, Signaling Gateway, Signal Transduction KE, and SuperArray. In addition, gene sets that have been extracted from experimental studies were included in the database. The curated gene sets were downloaded from MSigDB (version 4.0, C2). Because there was a chance of the biological pathway being narrowly defined, each pathway was set up to contain at least three genes in the following analyses. The names of gene sets were described with ‘brief description’ rather than ‘standard name’ which is available on the GSEA web (http://www.broadinstitute.org/gsea/index.jsp), because standard name equivocally explained function of gene set.
The statistical significance of the effects was estimated with a p-value less than 0.05 in both multivariate Cox-proportional hazard model by individual variants and polygenic risk score models and 0.1 in GSEA-SNP. The SAS statistical software package version 9.3, PLINK program version 1.07, and R 2.15.1 packages (GenABEL), STATA statistical software version 12.0 were used for the analyses.
Systematic review
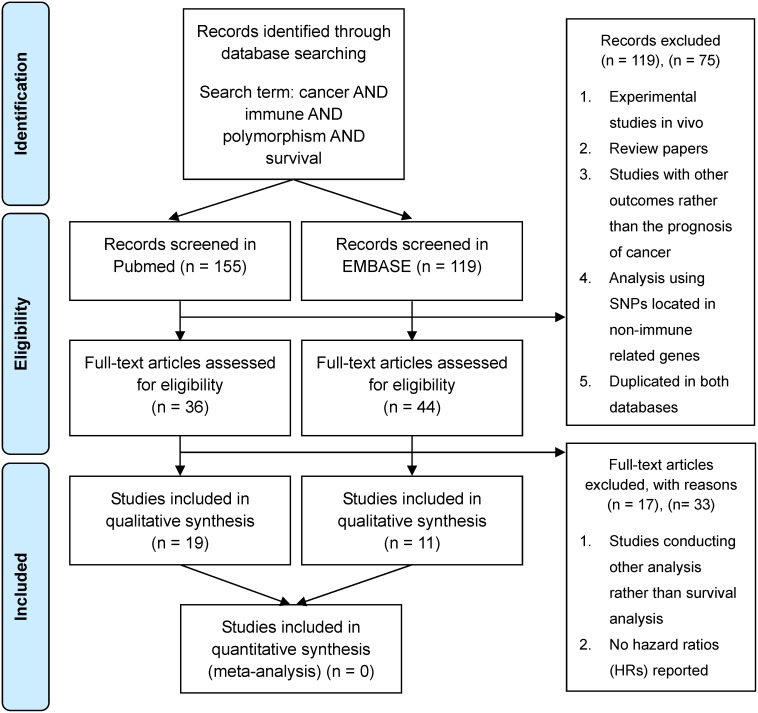
Previous studies conducting analyses to find associations between immunity-related genetic factors and the prognosis of cancer in the epidemiologic field were selected for Jan 2000 through Dec 2013 (Figure 2). Available studies for systematic review were searched in the PubMed and EMBASE database with a set of keywords that delineated breast cancer as well as other cancers, immune, genetic factors, and survival; cancer AND immune AND polymorphism AND survival. Abstracts were reviewed to identify reports examining associations between immunity-related genetic factors and clinical outcomes including recurrence and death. Literatures were excluded in the following circumstances; review paper, studies unrelated with genomic epidemiology, using SNPs located in non-immune related genes, duplicated in both databases, with no survival or recurrence data reported for survival analysis and no hazard ratios (HRs) reported which were estimated with the Cox-proportional hazard model for the associations of immunity-related genetic factors with cancer outcomes (Figure 2). In cases of duplication between both databases, the studies were deemed to have been searched in the PubMed database. The following data were extracted from each eligible study from the literature; disease site, authors, genes assessed, number of polymorphisms assessed, number of patients and events including recurrence, death, follow-up period, type of outcome, and covariates. Associations between polymorphisms and the outcome of each cancer were recorded as HR with 95% CI and adjustments. Because different nomenclatures and names for polymorphisms were used in the studies, all polymorphisms were named by RefSNP (rs) numbers. We followed the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement and checklist as a methodological template for this review (Table S1).
Figure 2. Overview of inclusion and exclusion criteria in systematic review.
Results
Table 1 shows the characteristics of the 107 patients including 20 patients who had the events. Among the 107 cases, BMI, PR status, and TNM stage showed a significant association with the prognosis on the DFS of breast cancer (P<0.05, log-rank test), while there were no significant differences in age, family history of breast cancer, educational level, menopausal status, smoking status, alcohol consumption, and ER status.
Table 1. Characteristic of study participants.
| Characteristics | No. of patients (%) | No. of events (%) | P a | HRb | (95% CI) | P b |
| Total | 107 (100.0) | 20 (100.0) | ||||
| Age (Mean ± SD) | 50.6±8.2 | 52.5±10.6 | 0.60 | |||
| <50 | 54 (50.5) | 11 (55.0) | 1.00 | |||
| ≥50 | 53 (49.5) | 9 (45.0) | 0.79 | (0.33–1.91) | 0.60 | |
| Body mass index (Mean ± SD) | 23.7±2.9 | 24.4±2.13 | <0.02 | |||
| <21.4 (median) | 30 (33.3) | 1 (5.0) | 1.00 | |||
| ≥21.4 | 77 (66.7) | 19 (95.0) | 7.30 | (0.98–54.61) | 0.05 | |
| Family history | 1.00 | |||||
| No | 97 (90.7) | 18 (90.0) | 1.00 | |||
| Yes | 10 (9.3) | 2 (10.0) | 1.00 | (0.23–4.37) | 1.00 | |
| Educational level | 0.46 | |||||
| ≤Middle school | 30 (28.3) | 4 (20.0) | 1.00 | |||
| High school | 46 (43.4) | 11 (55.0) | 1.95 | (0.62–6.13) | 0.26 | |
| ≥College or university | 30 (28.3) | 5 (25.0) | 1.27 | (0.34–4.73) | 0.72 | |
| Menopausal status | 0.71 | |||||
| Premenopausal | 62 (58.5) | 11 (55.0) | 1.00 | |||
| Postmenopausal | 44 (41.5) | 9 (45.0) | 1.18 | (0.49–2.84) | 0.72 | |
| Smoking status | 0.10 | |||||
| Never | 100 (93.5) | 17 (85.0) | ||||
| Ever | 7 (6.5) | 3 (15.0) | 2.70 | (0.78–9.17) | 0.12 | |
| Alcohol consumption | 0.66 | |||||
| Never | 70 (65.4) | 14 (70.0) | ||||
| Ever | 37 (34.6) | 6 (30.0) | 0.81 | (0.31–2.10) | 0.66 | |
| Estrogen receptor status | 0.07 | |||||
| Positive | 66 (62.3) | 9 (45.0) | 1.00 | |||
| Negative | 40 (37.7) | 11 (55.0) | 2.19 | (0.90–5.28) | 0.08 | |
| Progesterone receptor status | 0.01 | |||||
| Positive | 53 (50.5) | 5 (25.0) | 1.00 | |||
| Negative | 52 (49.5) | 15 (75.0) | 3.39 | (1.23–9.37) | 0.02 | |
| TNM stage | <0.01 | |||||
| 0/I | 48 (45.3) | 4 (20.0) | 1.00 | |||
| II | 40 (37.7) | 7 (35.0) | 2.20 | (0.64–7.56) | 0.21 | |
| III | 18 (17.0) | 9 (50.0) | 8.54 | (2.62–27.88) | <0.01 |
Log rank test.
Univariate Cox-proportional hazard model.
The associations of immunity-related genetic factors on DFS of breast cancer prognosis are presented in Table 2. Among 1,971 SNPs, 80 SNPs were significantly associated with the DFS of breast cancer. The 62 SNPs were remained after excluding those with high LD (r2>0.4) and 3 SNPs were still significant at FDR p-value less than 0.05. The SNPs were rs1952438 in SOCS4 gene (HR = 11.99, 95% CI = 3.62–39.72, P = 4.84E-05), rs2289278 in TSLP gene (HR = 4.25, 95% CI = 2.10–8.62, P = 5.99E-05) and rs2074724 in HGF gene (HR = 4.63, 95% CI = 2.18–9.87, P = 7.04E-05).
Table 2. Associations between the genetic variations of immunity-related genes and breast cancer disease free survival in the additive model (significance level, P<5.00E-02).
| Gene | Location | SNP | MAF | HRa | (95% CI) | P |
| SOCS4 | intronic | rs1952438 | 0.04 | 11.99 | (3.62–39.72) | 4.84E-05 |
| TSLP | UTR5 | rs2289278 | 0.15 | 4.25 | (2.10–8.62) | 5.99E-05 |
| HGF | intronic | rs2074724 | 0.11 | 4.63 | (2.18–9.87) | 7.04E-05 |
| IL-17C | intronic | rs2254073 | 0.15 | 4.24 | (1.90–9.49) | 4.31E-04 |
| BCL2 | intergenic | rs9989529 | 0.19 | 3.80 | (1.63–8.84) | 1.98E-03 |
| CCL2 | intergenic | rs17652343 | 0.08 | 4.57 | (1.74–11.97) | 2.01E-03 |
| ITGB2 | intronic | rs2838727 | 0.04 | 6.57 | (1.84–23.44) | 3.70E-03 |
| TRAF2 | intergenic | rs908831 | 0.14 | 3.79 | (1.54–9.36) | 3.79E-03 |
| NBN | downstream | rs2142097 | 0.42 | 3.55 | (1.48–8.49) | 4.40E-03 |
| SELE | intergenic | rs4656701 | 0.35 | 0.28 | (0.11–0.71) | 7.41E-03 |
| CCR1 | downstream | rs3136671 | 0.19 | 3.05 | (1.33–7.00) | 8.47E-03 |
| HGF | intronic | rs5745752 | 0.33 | 0.29 | (0.11–0.73) | 9.22E-03 |
| IL-12A | intergenic | rs9811792 | 0.31 | 0.23 | (0.08–0.71) | 1.01E-02 |
| MIF | ncRNA_exonic | rs1007888 | 0.41 | 2.39 | (1.22–4.67) | 1.11E-02 |
| ITGB2-AS1 | ncRNA_exonic | rs2070946 | 0.12 | 2.98 | (1.28–6.93) | 1.11E-02 |
| MIF | ncRNA_intronic | rs2000466 | 0.18 | 3.37 | (1.32–8.60) | 1.12E-02 |
| ALOXE3 | intronic | rs3027215 | 0.07 | 3.17 | (1.28–7.87) | 1.27E-02 |
| IFNAR2 | intronic | rs2073362 | 0.15 | 3.86 | (1.33–11.17) | 1.28E-02 |
| XDH | intergenic | rs10490361 | 0.46 | 0.44 | (0.23–0.84) | 1.35E-02 |
| CCL8 | intergenic | rs3138034 | 0.07 | 3.59 | (1.29–9.96) | 1.42E-02 |
| SOCS2 | intronic | rs3782415 | 0.48 | 2.38 | (1.18–4.83) | 1.60E-02 |
| DEF6 | intronic | rs6938946 | 0.34 | 2.26 | (1.16–4.39) | 1.68E-02 |
| ABHD16A | intronic | rs2295663 | 0.10 | 2.55 | (1.16–5.59) | 1.93E-02 |
| LBP | intronic | rs12624843 | 0.30 | 0.33 | (0.13–0.84) | 2.03E-02 |
| IL-18 | intergenic | rs243908 | 0.33 | 3.59 | (1.22–10.61) | 2.05E-02 |
| IL-10RB | UTR3 | rs1058867 | 0.32 | 2.62 | (1.14–6.04) | 2.33E-02 |
| IL-6R | intergenic | rs11265608 | 0.04 | 4.15 | (1.21–14.21) | 2.36E-02 |
| IRAK4 | intronic | rs4251460 | 0.11 | 2.78 | (1.15–6.73) | 2.38E-02 |
| TRAF5 | intronic | rs6684874 | 0.29 | 0.29 | (0.10–0.85) | 2.46E-02 |
| MIF | ncRNA_intronic | rs17004044 | 0.17 | 0.23 | (0.06–0.83) | 2.48E-02 |
| XDH | intronic | rs1429372 | 0.38 | 0.43 | (0.20–0.91) | 2.70E-02 |
| LMAN1 | intronic | rs12953981 | 0.41 | 0.41 | (0.19–0.91) | 2.74E-02 |
| ALOXE3 | intronic | rs3027208 | 0.43 | 0.44 | (0.21–0.91) | 2.76E-02 |
| CCL11 | intergenic | rs4795904 | 0.08 | 3.11 | (1.13–8.56) | 2.81E-02 |
| IL-12B | intergenic | rs4921468 | 0.22 | 2.54 | (1.10–5.87) | 2.85E-02 |
| IL-4R | UTR3 | rs8832 | 0.42 | 0.39 | (0.17–0.91) | 2.85E-02 |
| IL-12A | intergenic | rs747825 | 0.15 | 0.10 | (0.01–0.79) | 2.90E-02 |
| SCNN1A | intronic | rs3759324 | 0.36 | 2.10 | (1.07–4.14) | 3.03E-02 |
| ITGB2 | intronic | rs1474552 | 0.23 | 0.26 | (0.08–0.88) | 3.06E-02 |
| C6 | intronic | rs13168926 | 0.40 | 0.40 | (0.18–0.92) | 3.08E-02 |
| FGF2 | intergenic | rs308447 | 0.08 | 2.89 | (1.09–7.65) | 3.25E-02 |
| IL-10 | intronic | rs3021094 | 0.42 | 0.40 | (0.17–0.93) | 3.26E-02 |
| SELE | intergenic | rs4656699 | 0.20 | 0.31 | (0.11–0.92) | 3.41E-02 |
| STK19 | intronic | rs389883 | 0.26 | 1.96 | (1.05–3.67) | 3.46E-02 |
| STAT4 | intronic | rs1031509 | 0.31 | 0.43 | (0.19–0.94) | 3.53E-02 |
| NCF4 | intronic | rs2075938 | 0.39 | 2.17 | (1.05–4.51) | 3.66E-02 |
| SLC2A11 | intergenic | rs1984309 | 0.39 | 0.44 | (0.20–0.95) | 3.68E-02 |
| BPI | intronic | rs2275954 | 0.40 | 2.19 | (1.05–4.59) | 3.70E-02 |
| TNFRSF1A | intronic | rs4149577 | 0.41 | 0.37 | (0.14–0.94) | 3.70E-02 |
| KLK15 | upstream | rs3745523 | 0.29 | 2.07 | (1.04–4.12) | 3.81E-02 |
| BCL2 | intronic | rs12458289 | 0.28 | 2.27 | (1.04–4.96) | 4.00E-02 |
| MBL2 | intergenic | rs11003134 | 0.20 | 8.09 | (1.08–60.37) | 4.16E-02 |
| BCL10 | intergenic | rs6693365 | 0.30 | 2.36 | (1.03–5.39) | 4.18E-02 |
| SELE | intronic | rs3917412 | 0.28 | 2.13 | (1.03–4.40) | 4.21E-02 |
| CD180 | intergenic | rs6890674 | 0.15 | 2.27 | (1.03–5.02) | 4.29E-02 |
| MAL | intronic | rs3113002 | 0.35 | 0.45 | (0.21–0.98) | 4.30E-02 |
| AICDA | UTR3 | rs11046349 | 0.12 | 2.81 | (1.03–7.69) | 4.44E-02 |
| C1QA | intronic | rs2935542 | 0.14 | 2.29 | (1.02–5.13) | 4.49E-02 |
| IRF4 | intergenic | rs11242867 | 0.29 | 2.16 | (1.02–4.61) | 4.50E-02 |
| IL-8 | intergenic | rs4694178 | 0.40 | 0.48 | (0.23–0.99) | 4.61E-02 |
| MASP1 | intronic | rs3105782 | 0.15 | 2.30 | (1.01–5.24) | 4.70E-02 |
| MUC2 | intergenic | rs4077757 | 0.03 | 3.88 | (1.01–14.90) | 4.80E-02 |
Multivariate Cox proportional hazard model adjusted for age, estrogen receptor status, progesterone receptor status and TNM stage.
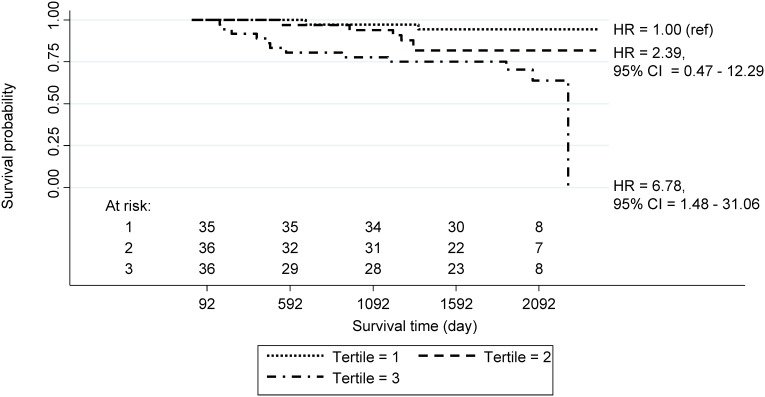
Figure 1 presents the Kaplan-Meier survival curve and estimated HRs of breast cancer in groups defined by tertile derived from the polygenic risk scores of the 107 patients with all 62 SNPs. The HR was significantly increased as the score increased (p for trend = 0.01). The HR of women in the 3rd tertile was 6.78 (95% CI = 1.48–31.06) compared to patients in the 1st tertile of polygenic risk score. Table 3 shows the predictive accuracy and validation results of polygenic risk score model. The Harrell’s C index of total patients is 0.813, and summarized Harrell’s C index of cross validation is 0.924.
Figure 1. Associations of the polygenic risk score on breast cancer disease free survival.
Kaplan-Meier survival curve and estimated hazard ratios (HRs) of breast cancer in groups defined by tertile derived from the polygenic risk scores of the 107 patients with all 62 SNPs.
Table 3. Harrell’s C index for polygenic risk score estimated by 4-fold cross-validation.
| Group | No. of SNPs in CV set | Harrell’s C index | Standard error | (95% CI) |
| All | 0.813 | 0.48 | (0.72–0.91) | |
| CV set1 | 25 | 0.885 | 0.09 | (0.70–1.07) |
| CV set2 | 40 | 0.910 | 0.06 | (0.78–1.04) |
| CV set3 | 32 | 0.940 | 0.03 | (0.88–1.00) |
| CV set4 | 36 | 0.909 | 0.04 | (0.82–1.00) |
| Summarya | 0.924 | 0.02 | (0.88–0.97) |
The summary of Harrell’s C index for 4 test sets calculated by fixed-effect meta-analysis.
In GSEA-SNP analysis, our results showed that 18 pathways with 62 SNPs in 56 immunity-related genes had significant association with the DFS of breast cancer at a p-value less than 0.1 (Table 4); set ‘Myc targets1’: targets of c-Myc identified by ChIP on chip in cultured cell lines, focusing on E-box-containing genes; high affinity bound subset (including BCL2 and NBN, P = 0.04), mitochondrial genes; based on literature and sequence annotation resources and converted to Affymetrix HG-U133A probe sets (including BCL2 and NBN, P = 0.04), genes down-regulated in T24 (bladder cancer) cells in response to the photodynamic therapy (PDT) stress (including BCL2 and CCL2, P = 0.04), genes transiently induced only by the second pulse of EGF in 184A1 cells (mammary epithelium) (including IRF3, TRAF5, KLK15 and IL5R, P = 0.02).
Table 4. Pathway analysis for immune related genes on breast cancer disease free survival using GSEA-SNP method (P<0.1).
| Included genes (No. of SNPs) | HRa | (95% CI) | Enrichment Score | Normal P b | Gene set (pathway) | Reference | |
| BCL2 (2), NBN (1) | 2.65 | (1.69–4.14) | 0.8594 | 0.04 | Set ‘Myc targets1’: targets of c-Myc identified byChIP on chip in cultured cell lines, focusing onE-box-containing genes; high affinity bound subset | Benporath et al. | [36] |
| Mitochondrial genes; based on literature and sequenceannotation resources and converted to AffymetrixHG-U133A probe sets | Mootha et al. | [43] | |||||
| BCL2 (2), CCL2 (1) | 3.12 | (1.98–4.90) | 0.8438 | 0.04 | Genes down-regulated in T24 (bladder cancer) cells inresponse to the photodynamic therapy (PDT) stress | Buytaert et al. | [29] |
| TSLP (1), BCL (2), TRAF5 (1), MASP1 (1) | 2.29 | (1.59–3.32) | 0.7392 | 0.06 | Genes down-regulated in prostate cancer samples | Liu et al. | [44] |
| SOCS4 (1), HGF (2) | 2.39 | (1.73–3.29) | 0.8552 | 0.07 | Human environmental stress response genes notchanged in primary fibroblasts from Wilmorsyndrom (WS) patients in response to 4NQO treatment | Kyng et al. | [35] |
| Human environmental stress response genes notchanged in primary fibroblasts from old donors inresponse to UV radiation | Kyng et al. | [35] | |||||
| TSLP (1), ALOXE3 (2), BCL2 (2), MAL (1), IRF4 (1) | 1.94 | (1.49–2.52) | 0.7163 | 0.08 | Set ‘H3K27 bound’: genes posessing the trimethylatedH3K27 (H3K27me3) mark in their promoters inhuman embryonic stem cells, as identified by ChIPon chip. | Benporath et al. | [36] |
| TSLP (1), ALOXE3 (2), BCL2 (2), MAL (1) | 1.99 | (1.52–2.62) | 0.7393 | 0.08 | Set ‘Suz12 targets’: genes identified by ChIP onchip as targets of the Polycomb protein SUZ12 inhuman embryonic stem cells. | Benporath et al. | [36] |
| HGF (2), BCL2 (2) | 2.48 | (1.71–3.60) | 0.7734 | 0.09 | Focal adhesion | KEGG | [45] |
| 0.7734 | 0.09 | Direct p53 effectors | PID | [46] | |||
| BCL2 (2), LBP (1) | 2.57 | (1.66–3.97) | 0.8136 | 0.09 | Genes in the expression cluster ‘Early ProgenitorsShared’: up-regulated in hematopoietic progenitorsfrom adult bone marrow and from fetal liver. | Ivanova et al. | [47] |
| TSLP (1), BCL2 (2), MAL (1) | 2.14 | (1.58–2.88) | 0.7617 | 0.10 | Set ‘EED targets’: genes identified by ChIP on chip as targets of the Polycomb protein EED in human embryonic stem cells. | Benporath et al. | [36] |
| 0.7617 | 0.10 | Set ‘PRC2 targets’: Polycomb Repression Complex 2(PRC) targets; identified by ChIP on chip on humanembryonic stem cells as genes that: posess thetrimethylated H3K27 mark in their promoters and are bound by SUZ12 and EED Polycomb proteins. | Benporath et al. | [36] | |||
| IRF3 (1), TRAF5 (1), KLK15 (1), IL4R (1) | 0.75 | (0.48–1.16) | −0.7414 | 0.02 | Genes transiently induced only by the second pulse ofEGF in 184A1 cells (mammary epithelium). | Zwang et al. | [48] |
| MBL2 (1), MASP1 (1), C6 (1) | 2.80 | (1.53–5.14) | −0.8438 | 0.06 | Lectin Induced Complement Pathway | Biocarta | [49] |
| −0.8438 | 0.06 | Genes down-regulated in liver samples of liver-specificknockout of HNF4A | Ohguchi et al. | [50] | |||
| IRF4 (1), TRAF5 (1), MUC (1) | 0.56 | (0.24–1.29) | −0.8136 | 0.07 | Genes up-regulated in the HMEC cells (primarymammary epithelium) upon expression of TP53 offadenoviral vector. | Perez et al. | [51] |
| CCR1 (1), IL8 (1), TNFRSF1A (1) | 0.72 | (0.43–1.20) | −0.7188 | 0.09 | Genes up-regulated in circulating endothelial cells(CEC) from cancer patients compared to those fromhealthy donors | Smirrnov et al. | [52] |
aMutivariate Cox proportional hazard model adjusted for age, hormone status and TNM stage according to polygenic risk score estimated by using SNPs included in each pathway.
P value for GSEA-SNP analysis.
Table 5 showed 30 studies resulted from systematic review for survival analyses estimating effects of immune-related genetic factors on various cancers. In the studies, eighty eight SNPs in 58 immunity genes were significantly associated with the prognosis of cancer patients (Table 6). In those results, there were 29 genes overlapped in both our study and previous studies, but no SNPs overlapped. Among them, IL-6R, IL-8, IL-10RB, IL-12A, and IL-12B was significantly associated with the prognosis of cancer consistent to our finding.
Table 5. Characteristics of previous studies.
| Types of cancer | Study authors | Genes assessed | No. of SNPs assessed | No. of patients | No. of events | Follow-up period, yrs | Types of outcomej | Adjusted covariatesk | Ref |
| Breast | Yang et al. | TLR4 | 4 | 604 | - | 4.9 | OS | - | [11] |
| You et al. | IL-21 | 4 | 891 | 121 | 5.0 | OS | age, age at menarche (years), menstrual status, BMI, pathological type, stage, ER status, PR status, family history of any cancer | [12] | |
| Hu et al. | IL-2 | 2 | 638 | - | 5.0 | OS | - | [13] | |
| DeMichele et al. | IL-6 | 4 | 346 | - | 11.2 | DFS | age at diagnosis, race, CYP3A4, GSTM1 | [14] | |
| Bewick et al. | ERCC1 and ERCC2 | 3 | 95 | 91 | 0.9 (PFS) 1.9 (BCSS) | PFS, BCSS | age | [53] | |
| Colorectal | Lu et al. | REG4, BML, and CD209 | 15 | 414 | 203 | 4.7 | OS | age at diagnosis, gender, TNM stage. | [54] |
| Castro et al. | 13 immune genes | 19 | 582 | 150 | 13.0 | OS | age at diagnosis, T, N stage. | [55] | |
| Slattery et al. | 11 immune genes | 50 | 1555a | 309 | >5.0 | OS | age, study center, ethnic group/ethnicity, sex, TNM stage, tumor molecular phenotype | [56] | |
| 754b | 171 | >5.0 | OS | ||||||
| Bondurant et al. | 13 immune genes | 59 | 1956a | 309 | >5.0 | OS | age, study center, ethnic group/ethnicity, sex, AJCC stage and tumor molecular phenotype | [57] | |
| 954b | 171 | >5.0 | OS | ||||||
| Non-small cell lung | Bi et al. | Cox-2 | 5 | 136 | - | 5.0 | OS | age, sex, smoking status, KPS, weight loss, histology, clinical stages, chemotherapy, radiation dosage | [58] |
| Dai et al. | 52 immune genes | 178 | 568 | 311 | 6.0 | OS | smoking status, histology, stage, surgical operation, chemotherapy, or radiotherapy status | [59] | |
| Sung et al. | FasL | 1 | 385 | 124 | 2.6i | OS, RFS | age, gender, smoking, tumor type, stage | [60] | |
| Yuan et al. | TGF-β1 | 3 | 205 | - | 1.4i | OS, DMFS | age, sex, race, KPS, smoking status, tumor histology, gross tumor volume, disease stage, receipt of chemotherapy or concurrent radiochemotherapy, number of cycles of chemotherapy, and radiation dose received | [61] | |
| Xue et al. | TGF-β1 | 2 | 109 | 85 | 1.2i | OS | age, gender, smoking status, histology, stage, radiation technique, radiation dose, and chemotherapy | [62] | |
| Schabath et al. | 53 inflammation-related genes | 326 | 651 | - | 2.1i | OS | age, gender, race, smoking status, stage, histology and first-course treatment. | [63] | |
| Guan et al. | TNF-α and TNFRSF1B | 5 | 225 | 155 | 1.9 | OS | age, gender, ethnicity, smoking status, tumor histology, KPS, tumor stage, node status, application of chemotherapy and radiotherapy dose | [64] | |
| Pine et al. | MBL2 | 5 | 558 (white population) | 405 | 3.8 | OS | sex, stage (III–IV versus I-II), age at diagnosis, current smoking status, and pack-years of smoking | [65] | |
| Bladder | Guirado et al. | C13ORF31, NOD2, TLR10, and RIPK2 | 5 | 349 | 66 | 3.9i | OS | - | [66] |
| Renal cell carcinoma | Schutz et al. | 70 immune genes | 290 | 403c | 184 | 5.3 | RFS | ECOG performance status, clinical stage, tumour size, tumour Fuhrman grade, histology (clear cell vs non clear cell) | [67] |
| 151c | 44 | 8.8 | RFS | ||||||
| Lymphoma | Aschebro-okkilfoy et al. | 40 immune genes | 82 | 496 | 211 | 12.0 | OS | age, education, stage, B-symptom, initial treatment. | [68] |
| Charbonneau et al. | 30 immune genes | 167 | 107d | 60 | 8.3 | EFS | clinical risk score, which accounts for the effects of treatment type and FLIPI (FL) or IPI (DLBCL) | [69] | |
| 82e | 39 | 8.3 | EFS | ||||||
| Habermann et al. | 44 immune genes | 73 | 365 | 96 | 4.8 | OS | age and clinical and demographic factors. | [70] | |
| Cerhan et al. | 44 immune genes | 73 | 278 | 59 | 4.8 | OS | age, clinical, demographic factors | [42] | |
| Melanoma | Lenci et al. | 15 type IFN genes | 44 | 625 | 174 | - | OS, DFS | gender, age and Breslow thickness | [71] |
| Ovarian | Goode et al. | 54 immune genes | 1536 | 3665 | 1529 | 5.4 | OS | study site, tumor stage, race, tumorgrade | [72] |
| Pancreatic | Reid-Lombardo et al. | 102 inflammatory genes | 1536 | 400f | 318 | 2.0i | OS | age, sex, body mass index class, stage, margin status (R0, R1, R2), grade, tumor size, and lymph node status | [73] |
| 443g | 420 | 0.8i | OS | ||||||
| 465h | 454 | 0.6i | OS | ||||||
| Osteosarcoma | Biason et al. | XPD, XPG, and XPA | 5 | 130 | 57 | 3.0 | EFS | covariate which were significant in the univariate analysis | [74] |
| Esophageal | Lee et al. | ERCC2 and ERCC4 | 2 | 400 | 310 | - | OS, PFS | T stage, N stage, Cell type, esophagectomy, CCRT | [75] |
| Head and neck | Lundberg et al. | TGF-β1 | 1 | 34 | 14 | 4.0 | OS, DFS | age, sex, cisplatin dose (mg/m2), RT dose (Gy) and treatment modality | [76] |
| Myeloma | Vangsted et al. | IL-1β, IL-6, IL-10, PPARγ2, and COX-2 | 6 | 348 | 68 | - | OS | β2-microglobulin, creatinine and Durie–Salmon stage | [77] |
Colon cancer patients, bRectal cancer patients, c403 cases are discovery cohort and 151 cases are validation cohort, each cohort selected from different center, dFollicular lymphoma patients, eDiffuse large B-cell lymphoma patients, fPatients who had undergone pancreatic resection operation, gPatients whose cancer locally advanced, hPatients whose cancer had metastasized, iMedian survival time, jOS, overall survival; DFS, disease free survival; RFS, relapse free survival; EFS, event free survival; DMFS, distant metastasis-free survival; BCSS, breast cancer specific survival, kAJCC, american joint committee on cancer; KPS, karnofski performance status; ECOG, eastern cooperative oncology group; CCRT, concurrent neoadjuvant chemoradiotherapy; FIGO, international federation of gynecology and obstetric.
Table 6. Genes that have significant SNPs of each study in the review of previous studies.
| Gene | SNP | Primary endpointa | HR | (95% CI) | P | Type of cancerb | Ref |
| C7 | rs324058 | EFS | 1.66 | (0.87–3.17) | 0.04 | Lymphoma | [69] |
| C9 | rs1421094 | EFS | 0.54 | (0.32–0.90) | 0.02 | Lymphoma | [69] |
| CCR5 | rs1800940 | OS | 0.73 | (0.53–1.00) | - | Lymphoma | [68] |
| CD46 | rs2466571 | EFS | 1.49 | (0.86–2.61) | 0.05 | Lymphoma | [69] |
| CD55 | rs2564978 | EFS | 0.52 | (0.30–0.88) | <0.01 | Lymphoma | [69] |
| CD80 | rs13071247 | OS | 1.73 | (1.26–2.39) | <0.01 | Ovarian cancer | [72] |
| rs7804190 | OS | 1.14 | (1.06–1.23) | <0.01 | Ovarian cancer | [72] | |
| CFH | rs3766404 | EFS | 2.25 | (1.31–3.87) | <0.01 | Lymphoma | [69] |
| rs1329423 | EFS | 0.49 | (0.29–0.38) | <0.01 | Lymphoma | [69] | |
| CFHR1 | rs436719 | EFS | 0.57 | (0.34–0.96) | 0.03 | Lymphoma | [69] |
| CFHR5 | rs6694672 | EFS | 2.63 | (1.41–4.92) | <0.01 | Lymphoma | [69] |
| CLU | rs3087554 | EFS | 0.46 | (0.21–1.00) | 0.05 | Lymphoma | [69] |
| COX-2 | rs689466 | OS | 0.58 | (0.39–0.86) | 0.01 | NSCLC | [58] |
| ERCC2 | rs238406 | OS | 1.64 | (1.08–2.50) | 0.02 | Esophageal cancer | [75] |
| rs238406 | PFS | 1.76 | (1.17–2.66) | 0.01 | Esophageal cancer | [75] | |
| rs1799793 | BCSS | 1.90 | (1.06–3.26) | 0.04 | Breast cancer | [53] | |
| rs1799793 | EFS | 0.23 | (0.05–0.99) | 0.01 | Osteosarcoma | [74] | |
| FasL | rs763110 | OS | 1.46 | (1.13–1.87) | <0.01 | NSCLC | [60] |
| rs763110 | RFS | 1.71 | (1.33–2.21) | <0.01 | NSCLC | [60] | |
| GATA3 | rs10905278 | OS | 1.82 | (1.31–2.53) | <0.01 | Pancreatic cancer | [73] |
| IFNAR1 | rs2257167 | EFS | 0.74 | (0.55–1.00) | 0.05 | NSCLC | [63] |
| IFNGR1 | rs1327474 | OS | 0.69 | (0.50–0.94) | 0.02 | Colorectal cancer | [56] |
| rs9376267 | OS | 1.37 | (1.09–1.73) | 0.01 | Colorectal cancer | [56] | |
| IFNGR2 | rs2834211 | OS | 1.32 | (1.01–1.72) | 0.04 | Colorectal cancer | [56] |
| rs2834213 | OS | 2.04 | (1.16–3.57) | 0.01 | Colorectal cancer | [56] | |
| IFNW1 | rs10964859 | OS | 1.80 | (1.02–3.16) | 0.04 | Melanoma | [71] |
| IL-10RB | rs8128184 | EFS | 1.59 | (1.11–2.29) | 0.01 | NSCLC | [63] |
| IL-12A | rs2243148 | EFS | 1.28 | (1.03–1.58) | 0.03 | NSCLC | [63] |
| IL-12B | rs3212227 | OS | 1.83 | (1.09–3.06) | <0.01 | Lymphoma | [42] |
| IL-13 | rs1295683 | EFS | 1.39 | (1.03–1.87) | 0.03 | NSCLC | [63] |
| IL-1A | rs3783546 | OS | 2.07 | (1.28–3.36) | 0.02 | Colorectal cancer | [57] |
| rs1800587 | OS | 1.90 | (1.26–2.87) | <0.01 | Lymphoma | [70] | |
| IL-1B | rs1143623 | OS | 1.37 | (1.09–1.72) | 0.01 | Colorectal cancer | [57] |
| rs1143627 | OS | 0.50 | (0.30–1.00) | 0.04 | Myeloma | [77] | |
| IL-1RN | rs454078 | OS | 1.93 | (1.11–3.34) | 0.03 | Lymphoma | [42] |
| IL-2 | rs2069763 | OS | 1.43 | (1.15–3.82) | - | Breast cancer | [13] |
| rs2069762 | OS | 1.80 | (1.06–3.05) | 0.01 | Lymphoma | [42] | |
| IL-21 | rs12508721 | OS | 0.45 | (0.30–0.67) | <0.01 | Breast cancer | [12] |
| IL-23R | rs6682925 | OS | 1.34 | (1.05–1.70) | - | NSCLC | [59] |
| IL-3 | rs181781 | OS | 2.47 | (1.11–5.53) | 0.03 | Colorectal cancer | [57] |
| IL-5 | rs2069807 | OS | 4.56 | (1.98–10.5) | <0.01 | Lymphoma | [70] |
| rs2069818 | OS | 5.58 | (1.66–18.6) | 0.01 | Lymphoma | [42] | |
| IL-5R | rs11713419 | OS | 6.60 | (2.42–18.02) | - | NSCLC | [59] |
| IL-6 | rs1800796 | OS | 0.42 | (0.23–0.77) | - | Lymphoma | [68] |
| rs1800797 | DFS | 1.60 | (1.09–2.35) | 0.02 | Breast cancer | [14] | |
| IL-6R | rs4240872 | EFS | 0.75 | (0.59–0.95) | 0.02 | NSCLC | [63] |
| IL-8 | rs4073 | OS | 2.14 | (1.26–3.63) | - | Lymphoma | [42] |
| rs2227307 | OS | 1.90 | (1.12–3.22) | - | Lymphoma | [42] | |
| rs2227306 | OS | 1.96 | (1.07–3.28) | - | Lymphoma | [42] | |
| rs12506479 | EFS | 1.32 | (1.08–1.62) | 0.01 | NSCLC | [63] | |
| IL-8RB | rs1126579 | OS | 1.61 | (1.05–2.46) | 0.02 | Colorectal cancer | [57] |
| rs1126580 | OS | 2.11 | (1.28–3.50) | <0.01 | Lymphoma | [70] | |
| IRF2 | rs12504466 | OS | 1.51 | (1.14–1.99) | <0.01 | Colorectal cancer | [56] |
| rs13116389 | OS | 1.38 | (1.09–1.75) | 0.01 | Colorectal cancer | [56] | |
| rs2797507 | OS | 0.77 | (0.61–0.98) | 0.03 | Colorectal cancer | [56] | |
| rs3775582 | OS | 0.67 | (0.50–0.89) | 0.01 | Colorectal cancer | [56] | |
| rs7655800 | OS | 1.33 | (1.04–1.70) | 0.02 | Colorectal cancer | [56] | |
| rs793801 | OS | 1.39 | (1.01–1.91) | 0.04 | Colorectal cancer | [56] | |
| rs1425551 | OS | 1.50 | (1.03–2.18) | 0.04 | Colorectal cancer | [56] | |
| rs3756094 | OS | 0.36 | (0.20–0.66) | <0.01 | Colorectal cancer | [56] | |
| rs3822118 | OS | 1.47 | (1.08–2.01) | 0.02 | Colorectal cancer | [56] | |
| rs807684 | OS | 0.30 | (0.14–0.66) | <0.01 | Colorectal cancer | [56] | |
| rs1044873 | OS | 1.32 | (1.04–1.68) | 0.03 | Colorectal cancer | [56] | |
| rs305083 | OS | 1.31 | (1.04–1.65) | 0.02 | Colorectal cancer | [56] | |
| IRF6 | rs2013196 | OS | 1.29 | (1.02–1.63) | 0.03 | Colorectal cancer | [56] |
| LRRC32 | rs3781699 | OS | 2.32 | (1.45–3.71) | <0.01 | Ovarian cancer | [72] |
| rs7944357 | OS | 2.04 | (1.34–3.10) | <0.01 | Ovarian cancer | [72] | |
| MBL2 | rs7096206 | OS | 0.55 | (0.42–0.73) | <0.01 | NSCLC | [65] |
| MET | rs11762213 | RFS | 1.86 | (1.17–2.95) | 0.01 | Renal cell cancer | [67] |
| NFKB | rs7157810 | OS | 1.43 | (1.16–1.75) | <0.01 | Pancreatic cancer | [73] |
| NOD2 | rs9302752 | OS | 3.19 | (2.04–4.34) | - | Bladder cancer | [66] |
| NOS3 | rs1799983 | OS | 1.39 | (1.14–1.70) | <0.01 | Pancreatic cancer | [73] |
| REG4 | rs2994809 | DFS | 2.00 | (1.18–3.39) | 0.01 | Colorectal cancer | [54] |
| rs2994811 | OS | 1.35 | (1.02–1.78) | 0.03 | Colorectal cancer | [54] | |
| RGS1 | rs10921202 | OS | 2.93 | (1.77–4.84) | <0.01 | Ovarian cancer | [72] |
| RIPK1 | rs2326173 | OS | 1.44 | (1.20–1.74) | <0.01 | Pancreatic cancer | [73] |
| SOCS3 | rs8064821 | OS | 0.65 | (0.49–0.87) | <0.01 | Pancreatic cancer | [73] |
| STAT1 | rs12693591 | OS | 0.68 | (0.55–0.86) | <0.01 | Pancreatic cancer | [73] |
| TGF-β1 | rs10469 | OS | 1.46 | (1.01–2.11) | 0.04 | NSCLC | [61] |
| rs1982073 | DMFS | 1.59 | (1.01–2.50) | 0.05 | NSCLC | [61] | |
| rs1982073 | DFS | 3.23 | (1.19–8.77) | 0.02 | HNSCC | [76] | |
| rs1800469 | OS | 0.46 | (0.25–0.87) | 0.02 | NSCLC | [62] | |
| TGFBR1 | rs10512263 | EFS | 0.59 | (0.37–0.94) | 0.03 | NSCLC | [63] |
| rs868 | EFS | 1.28 | (1.01–1.61) | 0.04 | NSCLC | [63] | |
| TGFBR2 | rs2043136 | EFS | 0.74 | (0.58–0.95) | 0.02 | NSCLC | [63] |
| TLR1 | rs5743551 | OS | 0.78 | (0.62–0.97) | - | NSCLC | [59] |
| TLR10 | rs4129009 | OS | 0.49 | (0.18–0.80) | - | Bladder cancer | [66] |
| TLR3 | rs3775291 | OS | 1.93 | (1.14–3.28) | - | Colorectal cancer | [55] |
| rs3775291 | OS | 1.37 | (1.09–1.73) | - | NSCLC | [59] | |
| TLR4 | rs11536889 | OS | 1.38 | (1.09–3.12) | 0.02 | Breast cancer | [11] |
| TNFRSF10B | rs11785599 | EFS | 1.41 | (1.16–1.70) | <0.01 | NSCLC | [63] |
| TNFRSF1B | rs1061622 | OS | 0.38 | (0.15–0.94) | 0.04 | NSCLC | [64] |
| TNFRSF4 | rs3753348 | OS | 3.41 | (1.65–7.05) | <0.01 | Ovarian cancer | [72] |
EFS, event free survival; OS, overall survival; RFS, relapse free survival; DFS, disease free survival; DMFS, distant metastasis-free survival; BCSS, breast cancer specific survival.
DLBCL, diffuse large B-cell lymphoma; NSCLC, non-small cell lung cancer; FL, follicular lymphoma; HNSCC, head and neck squamous cell carcinoma.
Discussion
In this study, we found that the rs1952438 in the suppressors of cytokine signaling (SOCS4) gene, rs2289278 in the thymic stromal lymphopoietin (TSLP) gene and rs2074724 in the hepatocyte growth factor (HGF) gene were highly associated with a poor prognosis of breast cancer. Moreover, the polygenic risk score model with genetic variations of immunity-related genes showed that the hazard of DFS of patients was significantly increased as high-risk alleles accumulated. In the GSEA-SNP analysis, 18 pathways significantly affected breast cancer prognosis.
The rs1952438 is located in the intron region of SOCS4 gene. SOCS family are rapidly induced by activated STATs and negatively regulate JAK/STAT pathway by a classical feedback loop [22]. Furthermore, other signal molecules such as FAK, IRS, p65, GR which are related with carcinogenesis, are regulated by SOCS proteins [23]–[27]. In addition, there are several previous study which reported that people who have higher expression level of SOCS4 are likely remained disease free status compared to those who developed recurrence [28]. In the view of previous studies which explain functional importance of SOCS4 and results of present study, it might be assumed that rs1952438 is associated with poorer prognosis of breast cancer by declining expression level of SOCS4.
The rs2289278 is found in intron 2 of the long-form of TSLP and in the 5′ untranslated region of the short-form of TSLP [29]. TSLP is a member of the IL-2 cytokine family and a distant paralog of IL-7. TSLP may have an important role in tumor progression by activating CD4+ T cells, inducing the expressing of OX40L in dendritic cells (DCs), and producing Th2-type cytokines and B-cell growth factor [30]. A recent study has shown that breast cancer cells have high expression levels of TSLP, indicating that the TSLP may be critical in the development of breast cancer [31]. It is that high expression level of TSLP in cancer increases the Th2 level [30]. Furthermore, Th2 cytokines promote disease progression through the increased survival of cancer cells, M2 macrophage differentiation, and fibrosis [31], [32]. Thus, TSLP may be an important factor of breast tumor progression and the prognosis of a patient.
The rs2074724 is located in the intron of HGF. HGF is known to activate angiogenesis of tumors as well as cell-cell interactions, matrix adhesion, migration, invasion [33]. Moreover, breast cancer patients with a high HGF concentration had a significantly poor prognosis when compared to those with a low HGF concentration [34]. Therefore, HGF level was found to be the most important independent factor in predicting the prognosis of breast cancer.
In the GSEA-SNP analysis, there are 18 significant pathways; among these pathways, gene set from Kyng et al [35] which included rs1952438 in SOCS4 gene and rs2074724, and rs5745752 in HGF gene is described that environmental stress such as 4-nitroquinoline-1-oxide (4NQO) elicited DNA damage specific gene expression changes of up to 10. In short, it can be expected that those SNPs included in the pathway can up-regulate breast cancer progression and result in poor prognosis by influencing on environmental response, although there are not precise result in this assumption.
‘Myc tagets1’ gene set from Benporath et al [36] which included rs12458289 and rs9989529 in BCL2 gene, and rs2142097 in NBN gene is shown as the most significant gene set. Benporath et al describe that targets of Nanog, Oct4, Sox2 and c-Myc are more frequently associated in poorly differentiated tumors than in well-differentiated tumors. c-Myc is well known to directly regulate the expression of NBN gene involved in DNA double-strand break repair and can result in chromosomal instability, cellular proliferation defects leading to increased more aggressive and metastatic tumor latency [37], [38]. BCL2 and c-Myc are known to make the negative feedback loop in breast cancer cell line [39]. Taking all these consideration of both Benporath et al and results of present study to account, it is can be deduced that rs12458289, rs9989529, and rs2142097 might be associated with the prognosis of breast cancer by interacting with c-MYC gene.
To support the indirectly functional effects of our results, we attempted to find potential functional SNPs in SOCS4, HGF, TSLP and genes included in GSEA-SNP using UCSC database [40] and checked the LD between the potential functional SNPs and our findings. Table S2 show the functional SNPs studied in this study and functional SNPs in LD with those SNPs, generally to affect histone modification, DNA methylation, and binding affinity of several transcription factors located in 5′UTR or 3′UTR. For example, transcription activity of IL-8 is influenced by rs4073 which located in promoter region of IL-8 [41] and the variant increased the risk of mortality in follicular lymphocytic leukemia by increasing production of IL-8 [42]. As a result, it is possibly anticipated that those potential SNPs may influence to breast cancer prognosis by regulating the epigenetic and transcriptional pathway.
Several previous reports have evaluated the associations of immunity gene polymorphism and breast cancer prognosis [11]–[14]. They suggested that the variants of ERCC2, TLR4, IL-2, IL-6, and IL-21 genes had associations with breast cancer prognosis respectively. However, those genes were not replicated in present study. In the other types of cancer studies, IL-6R, IL-8, IL-10RB, IL-12A, and IL-12B genes were consistently associated with cancer prognosis between our study and theirs. However, there were few consistent SNPs with cancer prognosis in our review of the literature, which may result from various cancer targets, different ethnicities, and different prognostic factors in the models and statistical power.
In this study, there are several limitations including a small sample size and absence of an external validation study. Since the power of this study was low to detect accurate results, the results of this study are carefully interpreted, although the significance levels of top 3 SNPs passed the FDR test with significance (p<0.05) and the internal validity was confirmed by the cross-validation. In addition, polygenic risk score model and GSEA-SNP are conducted with whole significant SNPs which include insignificant SNPs at FDR p-value greater than 0.05. Tag SNPs selected based on the data of a Caucasian population and lack of breast cancer subtype information were also limitations of this study. In the systematic review-level, the summary measure and synthesis of the results were not calculated because various genes and the variations related to immune response were the focus. However, the strength of this study is that lots of genetic factors in immune-related genes were covered at once. Moreover, it attempted to apply the candidate gene approach to cover the pathway of immunity-related genetic factors with breast cancer prognosis in Asian women.
In conclusion, our study found that common variants in the SOCS4, TSLP and HGF genes might be related with breast cancer prognosis in Korean women. Hazard of DFS in patients was significantly increased when high-risk alleles were accumulated. Therefore, our results suggest that genetic polymorphisms in immunity-related genes have relevance to breast cancer prognosis among Korean women. Further large-scale functional studies are needed to confirm our findings.
Supporting Information
PRISMA checklist.
(DOCX)
Potential functional SNPs which has a LD with SNPs in SOCS4, HGF, TSLP and gene in GSEA-SNP (r2>0.8).
(DOCX)
Funding Statement
This research was supported by a grant of Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C0065). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jemal A (2012) Global burden of cancer: opportunities for prevention. Lancet 380: 1797–1799. [DOI] [PubMed] [Google Scholar]
- 2. Witz IP, Levy-Nissenbaum O (2006) The tumor microenvironment in the post-PAGET era. Cancer Lett 242: 1–10. [DOI] [PubMed] [Google Scholar]
- 3. Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, et al. (2013) Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat 45: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Key TJ, Verkasalo PK, Banks E (2001) Epidemiology of breast cancer. Lancet Oncol 2: 133–140. [DOI] [PubMed] [Google Scholar]
- 5. Sariego J, Zrada S, Byrd M, Matsumoto T (1995) Breast cancer in young patients. Am J Surg 170: 243–245. [DOI] [PubMed] [Google Scholar]
- 6. Zhang Y, Cheng S, Zhang M, Zhen L, Pang D, et al. (2013) High-infiltration of tumor-associated macrophages predicts unfavorable clinical outcome for node-negative breast cancer. PLoS One 8: e76147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dan T, Hewitt SM, Ohri N, Ly D, Soule BP, et al. (2014) CD44 is prognostic for overall survival in the NCI randomized trial on breast conservation with 25 year follow-up. Breast Cancer Res Treat 143: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mori K, Shibanuma M, Nose K (2004) Invasive potential induced under long-term oxidative stress in mammary epithelial cells. Cancer Res 64: 7464–7472. [DOI] [PubMed] [Google Scholar]
- 9. Lewis CE, Leek R, Harris A, McGee JO (1995) Cytokine regulation of angiogenesis in breast cancer: the role of tumor-associated macrophages. J Leukoc Biol 57: 747–751. [DOI] [PubMed] [Google Scholar]
- 10. Schreibelt G, Tel J, Sliepen KH, Benitez-Ribas D, Figdor CG, et al. (2010) Toll-like receptor expression and function in human dendritic cell subsets: implications for dendritic cell-based anti-cancer immunotherapy. Cancer Immunol Immunother 59: 1573–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang CX, Li CY, Feng W (2013) Toll-like receptor 4 genetic variants and prognosis of breast cancer. Tissue Antigens 81: 221–226. [DOI] [PubMed] [Google Scholar]
- 12. You Y, Deng J, Zheng J, Hu M, Li N, et al. (2013) IL-21 gene polymorphism is associated with the prognosis of breast cancer in Chinese populations. Breast Cancer Res Treat 137: 893–901. [DOI] [PubMed] [Google Scholar]
- 13. Hu XB, Ouyang LZ, Tang LL (2013) Interleukin-2 gene polymorphisms and prognosis of breast cancer. Genet Test Mol Biomarkers 17: 453–457. [DOI] [PubMed] [Google Scholar]
- 14. DeMichele A, Gray R, Horn M, Chen J, Aplenc R, et al. (2009) Host genetic variants in the interleukin-6 promoter predict poor outcome in patients with estrogen receptor-positive, node-positive breast cancer. Cancer Res 69: 4184–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee JY, Park AK, Lee KM, Park SK, Han S, et al. (2009) Candidate gene approach evaluates association between innate immunity genes and breast cancer risk in Korean women. Carcinogenesis 30: 1528–1531. [DOI] [PubMed] [Google Scholar]
- 16.Boyle P, Levin B, Cancer IAfRo (2008) World cancer report 2008: IARC Press.
- 17. Park SK, Yang JJ, Oh S, Cho LY, Ma SH, et al. (2012) Innate immunity and non-Hodgkin’s lymphoma (NHL) related genes in a nested case-control study for gastric cancer risk. PLoS One 7: e45274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological): 289–300. [Google Scholar]
- 19. Reeves GK, Travis RC, Green J, Bull D, Tipper S, et al. (2010) Incidence of breast cancer and its subtypes in relation to individual and multiple low-penetrance genetic susceptibility loci. Jama 304: 426–434. [DOI] [PubMed] [Google Scholar]
- 20. Harrell FE Jr, Lee KL, Mark DB (1996) Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15: 361–387. [DOI] [PubMed] [Google Scholar]
- 21. Holden M, Deng S, Wojnowski L, Kulle B (2008) GSEA-SNP: applying gene set enrichment analysis to SNP data from genome-wide association studies. Bioinformatics 24: 2784–2785. [DOI] [PubMed] [Google Scholar]
- 22. Hilton DJ (1999) Negative regulators of cytokine signal transduction. Cell Mol Life Sci 55: 1568–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Emanuelli B, Peraldi P, Filloux C, Chavey C, Freidinger K, et al. (2001) SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-alpha in the adipose tissue of obese mice. J Biol Chem 276: 47944–47949. [DOI] [PubMed] [Google Scholar]
- 24. Haffner MC, Jurgeit A, Berlato C, Geley S, Parajuli N, et al. (2008) Interaction and functional interference of glucocorticoid receptor and SOCS1. J Biol Chem 283: 22089–22096. [DOI] [PubMed] [Google Scholar]
- 25. Liu E, Cote JF, Vuori K (2003) Negative regulation of FAK signaling by SOCS proteins. Embo j 22: 5036–5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ryo A, Suizu F, Yoshida Y, Perrem K, Liou YC, et al. (2003) Regulation of NF-kappaB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Mol Cell 12: 1413–1426. [DOI] [PubMed] [Google Scholar]
- 27. Ueki K, Kondo T, Kahn CR (2004) Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol Cell Biol 24: 5434–5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sasi W, Jiang WG, Sharma A, Mokbel K (2010) Higher expression levels of SOCS 1, 3, 4, 7 are associated with earlier tumour stage and better clinical outcome in human breast cancer. BMC Cancer 10: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu W, Xu LS, Liu QJ, Dong FZ, Qiu RF, et al. (2012) Two single nucleotide polymorphisms in TSLP gene are associated with asthma susceptibility in Chinese Han population. Exp Lung Res 38: 375–382. [DOI] [PubMed] [Google Scholar]
- 30. Ziegler SF, Roan F, Bell BD, Stoklasek TA, Kitajima M, et al. (2013) The biology of thymic stromal lymphopoietin (TSLP). Adv Pharmacol 66: 129–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Olkhanud PB, Rochman Y, Bodogai M, Malchinkhuu E, Wejksza K, et al. (2011) Thymic stromal lymphopoietin is a key mediator of breast cancer progression. J Immunol 186: 5656–5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pedroza-Gonzalez A, Xu K, Wu TC, Aspord C, Tindle S, et al. (2011) Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J Exp Med 208: 479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Parr C, Watkins G, Mansel RE, Jiang WG (2004) The hepatocyte growth factor regulatory factors in human breast cancer. Clin Cancer Res 10: 202–211. [DOI] [PubMed] [Google Scholar]
- 34. Yamashita J, Ogawa M, Yamashita S, Nomura K, Kuramoto M, et al. (1994) Immunoreactive hepatocyte growth factor is a strong and independent predictor of recurrence and survival in human breast cancer. Cancer Res 54: 1630–1633. [PubMed] [Google Scholar]
- 35. Kyng KJ, May A, Stevnsner T, Becker KG, Kolvra S, et al. (2005) Gene expression responses to DNA damage are altered in human aging and in Werner Syndrome. Oncogene 24: 5026–5042. [DOI] [PubMed] [Google Scholar]
- 36. Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, et al. (2008) An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet 40: 499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chiang YC, Teng SC, Su YN, Hsieh FJ, Wu KJ (2003) c-Myc directly regulates the transcription of the NBS1 gene involved in DNA double-strand break repair. J Biol Chem 278: 19286–19291. [DOI] [PubMed] [Google Scholar]
- 38. Shimada M, Sagae R, Kobayashi J, Habu T, Komatsu K (2009) Inactivation of the Nijmegen breakage syndrome gene leads to excess centrosome duplication via the ATR/BRCA1 pathway. Cancer Res 69: 1768–1775. [DOI] [PubMed] [Google Scholar]
- 39. Gaire RK, Smith L, Humbert P, Bailey J, Stuckey PJ, et al. (2013) Discovery and analysis of consistent active sub-networks in cancers. BMC Bioinformatics 14 Suppl 2: S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, et al. (2002) The human genome browser at UCSC. Genome Res 12: 996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hull J, Thomson A, Kwiatkowski D (2000) Association of respiratory syncytial virus bronchiolitis with the interleukin 8 gene region in UK families. Thorax 55: 1023–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cerhan JR, Wang S, Maurer MJ, Ansell SM, Geyer SM, et al. (2007) Prognostic significance of host immune gene polymorphisms in follicular lymphoma survival. Blood 109: 5439–5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, et al. (2003) PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34: 267–273. [DOI] [PubMed] [Google Scholar]
- 44. Buytaert E, Matroule JY, Durinck S, Close P, Kocanova S, et al. (2008) Molecular effectors and modulators of hypericin-mediated cell death in bladder cancer cells. Oncogene 27: 1916–1929. [DOI] [PubMed] [Google Scholar]
- 45. Kanehisa M, Goto S, Sato Y, Kawashima M, Furumichi M, et al. (2014) Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic Acids Res 42: D199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schaefer CF, Anthony K, Krupa S, Buchoff J, Day M, et al. (2009) PID: the Pathway Interaction Database. Nucleic Acids Res 37: D674–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ivanova NB, Dimos JT, Schaniel C, Hackney JA, Moore KA, et al. (2002) A stem cell molecular signature. Science 298: 601–604. [DOI] [PubMed] [Google Scholar]
- 48. Zwang Y, Sas-Chen A, Drier Y, Shay T, Avraham R, et al. (2011) Two phases of mitogenic signaling unveil roles for p53 and EGR1 in elimination of inconsistent growth signals. Mol Cell 42: 524–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nishimura D (2001) BioCarta. Biotech Software & Internet Report: The Computer Software Journal for Scient 2: 117–120. [Google Scholar]
- 50. Ohguchi H, Tanaka T, Uchida A, Magoori K, Kudo H, et al. (2008) Hepatocyte nuclear factor 4alpha contributes to thyroid hormone homeostasis by cooperatively regulating the type 1 iodothyronine deiodinase gene with GATA4 and Kruppel-like transcription factor 9. Mol Cell Biol 28: 3917–3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Perez CA, Ott J, Mays DJ, Pietenpol JA (2007) p63 consensus DNA-binding site: identification, analysis and application into a p63MH algorithm. Oncogene 26: 7363–7370. [DOI] [PubMed] [Google Scholar]
- 52. Smirnov DA, Foulk BW, Doyle GV, Connelly MC, Terstappen LW, et al. (2006) Global gene expression profiling of circulating endothelial cells in patients with metastatic carcinomas. Cancer Res 66: 2918–2922. [DOI] [PubMed] [Google Scholar]
- 53. Bewick MA, Lafrenie RM, Conlon MSC (2011) Nucleotide excision repair polymorphisms and survival outcome for patients with metastatic breast cancer. Journal of Cancer Research and Clinical Oncology 137: 543–550. [DOI] [PubMed] [Google Scholar]
- 54. Lu S, Bevier M, Huhn S, Sainz J, Lascorz J, et al. (2013) Genetic variants in C-type lectin genes are associated with colorectal cancer susceptibility and clinical outcome. Int J Cancer. [DOI] [PubMed] [Google Scholar]
- 55. Castro FA, Forsti A, Buch S, Kalthoff H, Krauss C, et al. (2011) TLR-3 polymorphism is an independent prognostic marker for stage II colorectal cancer. Eur J Cancer 47: 1203–1210. [DOI] [PubMed] [Google Scholar]
- 56. Slattery ML, Lundgreen A, Bondurant KL, Wolff RK (2011) Interferon-signaling pathway: associations with colon and rectal cancer risk and subsequent survival. Carcinogenesis 32: 1660–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bondurant KL, Lundgreen A, Herrick JS, Kadlubar S, Wolff RK, et al. (2013) Interleukin genes and associations with colon and rectal cancer risk and overall survival. Int J Cancer 132: 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bi N, Yang M, Zhang L, Chen X, Ji W, et al. (2010) Cyclooxygenase-2 genetic variants are associated with survival in unresectable locally advanced non-small cell lung cancer. Clin Cancer Res 16: 2383–2390. [DOI] [PubMed] [Google Scholar]
- 59. Dai J, Hu Z, Dong J, Xu L, Pan S, et al. (2012) Host immune gene polymorphisms were associated with the prognosis of non-small-cell lung cancer in Chinese. Int J Cancer 130: 671–676. [DOI] [PubMed] [Google Scholar]
- 60. Sung WW, Wang YC, Cheng YW, Lee MC, Yeh KT, et al. (2011) A polymorphic −844T/C in FasL promoter predicts survival and relapse in non-small cell lung cancer. Clin Cancer Res 17: 5991–5999. [DOI] [PubMed] [Google Scholar]
- 61. Yuan X, Wei Q, Komaki R, Liu Z, Yang J, et al. (2013) TGF(beta)1 Polymorphisms Predict Distant Metastasis-Free Survival in Patients with Inoperable Non-Small-Cell Lung Cancer after Definitive Radiotherapy. PLoS ONE 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xue SL, Zheng YH, Su HF, Deng X, Zhang XB, et al. (2013) Association between single nucleotide polymorphisms of the transforming growth factor-beta1 gene and overall survival in unresectable locally advanced non-small-cell lung cancer patients treated with radio(chemo)therapy in a Chinese population. Medical Oncology 30. [DOI] [PubMed] [Google Scholar]
- 63. Schabath MB, Giuliano AR, Thompson ZJ, Amankwah EK, Gray JE, et al. (2013) TNFRSF10B polymorphisms and haplotypes associated with increased risk of death in non-small cell lung cancer. Carcinogenesis 34: 2525–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Guan X, Liao Z, Ma H, Qian J, Liu Z, et al. (2011) TNFRSF1B +676 T>G polymorphism predicts survival of non-Small cell lung cancer patients treated with chemoradiotherapy. BMC Cancer 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pine SR, Mechanic LE, Ambs S, Bowman ED, Chanock SJ, et al. (2007) Lung cancer survival and functional polymorphisms in MBL2, an innate-immunity gene. Journal of the National Cancer Institute 99: 1401–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Guirado M, Gil H, Saenz-Lopez P, Reinboth J, Garrido F, et al. (2012) Association between C13ORF31, NOD2, RIPK2 and TLR10 polymorphisms and urothelial bladder cancer. Hum Immunol 73: 668–672. [DOI] [PubMed] [Google Scholar]
- 67. Schutz FA, Pomerantz MM, Gray KP, Atkins MB, Rosenberg JE, et al. (2013) Single nucleotide polymorphisms and risk of recurrence of renal-cell carcinoma: a cohort study. Lancet Oncol 14: 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Aschebrook-Kilfoy B, Zheng T, Foss F, Ma S, Han X, et al. (2012) Polymorphisms in immune function genes and non-Hodgkin lymphoma survival. J Cancer Surviv 6: 102–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Charbonneau B, Maurer MJ, Fredericksen ZS, Zent CS, Link BK, et al. (2012) Germline variation in complement genes and event-free survival in follicular and diffuse large B-cell lymphoma. Am J Hematol 87: 880–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Habermann TM, Wang SS, Maurer MJ, Morton LM, Lynch CF, et al. (2008) Host immune gene polymorphisms in combination with clinical and demographic factors predict late survival in diffuse large B-cell lymphoma patients in the pre-rituximab era. Blood 112: 2694–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lenci RE, Bevier M, Brandt A, Bermejo JL, Sucker A, et al. (2012) Influence of genetic variants in type I interferon genes on melanoma survival and therapy. PLoS One 7: e50692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Goode EL, DeRycke M, Kalli KR, Oberg AL, Cunningham JM, et al. (2013) Inherited variants in regulatory T cell genes and outcome of ovarian cancer. PLoS One 8: e53903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Reid-Lombardo KM, Fridley BL, Bamlet WR, Cunningham JM, Sarr MG, et al. (2013) Survival is associated with genetic variation in inflammatory pathway genes among patients with resected and unresected pancreatic cancer. Annals of Surgery 257: 1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Biason P, Hattinger CM, Innocenti F, Talamini R, Alberghini M, et al. (2012) Nucleotide excision repair gene variants and association with survival in osteosarcoma patients treated with neoadjuvant chemotherapy. Pharmacogenomics Journal 12: 476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lee JM, Yang PW, Yang SY, Chuang TH, Tung EC, et al. (2011) Genetic variants in DNA repair predicts the survival of patients with esophageal cancer. Annals of Surgery 253: 918–927. [DOI] [PubMed] [Google Scholar]
- 76. Lundberg M, Saarilahti K, Makitie AA, Mattila PS (2010) TGF(beta)1 genetic polymorphism is associated with survival in head and neck squamous cell carcinoma independent of the severity of chemoradiotherapy induced mucositis. Oral Oncology 46: 369–372. [DOI] [PubMed] [Google Scholar]
- 77. Vangsted AJ, Klausen TW, Ruminski W, Gimsing P, Andersen NF, et al. (2009) The polymorphism IL-1(beta) T-31C is associated with a longer overall survival in patients with multiple myeloma undergoing auto-SCT. Bone Marrow Transplantation 43: 539–545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist.
(DOCX)
Potential functional SNPs which has a LD with SNPs in SOCS4, HGF, TSLP and gene in GSEA-SNP (r2>0.8).
(DOCX)