Abstract
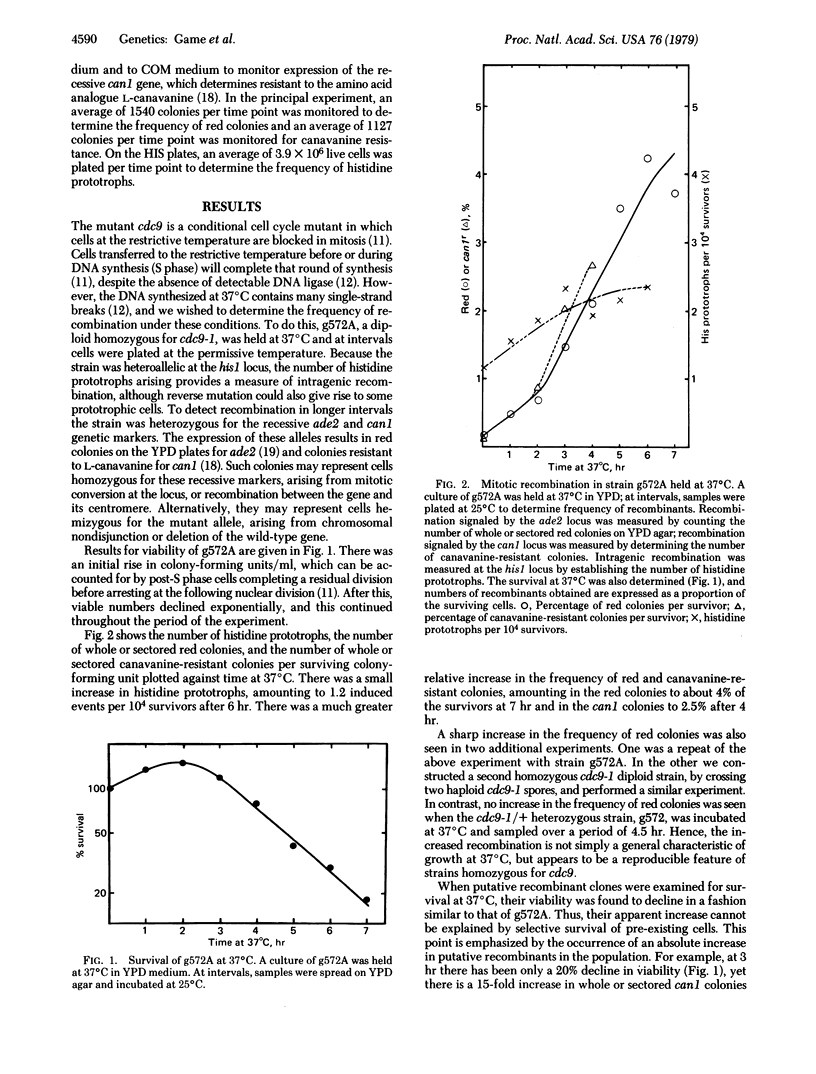
The temperature-sensitive Saccharomyces cerevisiae cell cycle mutant cdc9 is defective in DNA ligase, and the DNA synthesized at the restrictive temperature contains many single-strand breaks. We find that holding a diploid homozygous for cdc9 at the restrictive temperature and then plating cells at the permissive temperature gives rise to increased intragenic and intergenic recombination. In the latter case, recombinants signaled by the ade2 locus rise to about 4% of the survivors after 6 hr of incubation at the restrictive temperature. We propose that the single-strand breaks left in DNA synthesized at the restrictive temperature may lead to recombination.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boram W. R., Roman H. Recombination in Saccharomyces cerevisiae: a DNA repair mutation associated with elevated mitotic gene conversion. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2828–2832. doi: 10.1073/pnas.73.8.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox B., Game J. Repair systems in Saccharomyces. Mutat Res. 1974 Aug;26(4):257–264. doi: 10.1016/s0027-5107(74)80023-0. [DOI] [PubMed] [Google Scholar]
- Culotti J., Hartwell L. H. Genetic control of the cell division cycle in yeast. 3. Seven genes controlling nuclear division. Exp Cell Res. 1971 Aug;67(2):389–401. doi: 10.1016/0014-4827(71)90424-1. [DOI] [PubMed] [Google Scholar]
- Esposito M. S. Evidence that spontaneous mitotic recombination occurs at the two-strand stage. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4436–4440. doi: 10.1073/pnas.75.9.4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortuin J. J. Another two genes controlling mitotic intragenic recombination and recovery from UV damage in Aspergillus nidulans. II. Recombination behaviour and x-ray-sensitivity of uvsD and uvsE mutants. Mutat Res. 1971 Mar;11(3):265–277. [PubMed] [Google Scholar]
- Game J. C., Mortimer R. K. A genetic study of x-ray sensitive mutants in yeast. Mutat Res. 1974 Sep;24(3):281–292. doi: 10.1016/0027-5107(74)90176-6. [DOI] [PubMed] [Google Scholar]
- Golin J. E., Esposito M. S. Evidence for joint genic control of spontaneous mutation and genetic recombination during mitosis in Saccharomyces. Mol Gen Genet. 1977 Jan 18;150(2):127–135. doi: 10.1007/BF00695392. [DOI] [PubMed] [Google Scholar]
- Grenson M., Mousset M., Wiame J. M., Bechet J. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. I. Evidence for a specific arginine-transporting system. Biochim Biophys Acta. 1966 Oct 31;127(2):325–338. doi: 10.1016/0304-4165(66)90387-4. [DOI] [PubMed] [Google Scholar]
- Holliday R., Halliwell R. E., Evans M. W., Rowell V. Genetic characterization of rec-1, a mutant of Ustilago maydis defective in repair and recombination. Genet Res. 1976 Jun;27(3):413–453. doi: 10.1017/s0016672300016621. [DOI] [PubMed] [Google Scholar]
- Jansen G. J. Abnormal frequencies of spontaneous mitotic recombination in uvsB and uvsC mutants of Aspergillus nidulans. Mutat Res. 1970 Jul;10(1):33–41. doi: 10.1016/0027-5107(70)90143-0. [DOI] [PubMed] [Google Scholar]
- Johnston L. H., Nasmyth K. A. Saccharomyces cerevisiae cell cycle mutant cdc9 is defective in DNA ligase. Nature. 1978 Aug 31;274(5674):891–893. doi: 10.1038/274891a0. [DOI] [PubMed] [Google Scholar]
- Konrad E. B. Method for the isolation of Escherichia coli mutants with enhanced recombination between chromosomal duplications. J Bacteriol. 1977 Apr;130(1):167–172. doi: 10.1128/jb.130.1.167-172.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClary D. O., Nulty W. L., Miller G. R. EFFECT OF POTASSIUM VERSUS SODIUM IN THE SPORULATION OF SACCHAROMYCES. J Bacteriol. 1959 Sep;78(3):362–368. doi: 10.1128/jb.78.3.362-368.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullaney P. D., Cox D. F. Sex proportion in swine after paternal X-irradiation. Mutat Res. 1969 May-Jun;7(3):482–484. doi: 10.1016/0027-5107(69)90123-7. [DOI] [PubMed] [Google Scholar]
- PONTECORVO G., KAFER E. Genetic analysis based on mitotic recombination. Adv Genet. 1958;9:71–104. [PubMed] [Google Scholar]
- Parag Y., Parag G. Mutations affecting mitotic recombination frequency in haploids and diploids of the filamentous fungus Aspergillus nidulans. Mol Gen Genet. 1975;137(2):109–123. doi: 10.1007/BF00341677. [DOI] [PubMed] [Google Scholar]
- Pauling C., Hamm L. Properties of a temperature-sensitive radiation-sensitive mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1495–1502. doi: 10.1073/pnas.60.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash S., Prakash L. Increased spontaneous mitotic segregation in MMS-sensitive mutants of Saccharomyces cerevisiae. Genetics. 1977 Oct;87(2):229–236. doi: 10.1093/genetics/87.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROMAN H. Studies of gene mutation in Saccharomyces. Cold Spring Harb Symp Quant Biol. 1956;21:175–185. doi: 10.1101/sqb.1956.021.01.015. [DOI] [PubMed] [Google Scholar]
- Radding C. M. Genetic recombination: strand transfer and mismatch repair. Annu Rev Biochem. 1978;47:847–880. doi: 10.1146/annurev.bi.47.070178.004215. [DOI] [PubMed] [Google Scholar]


