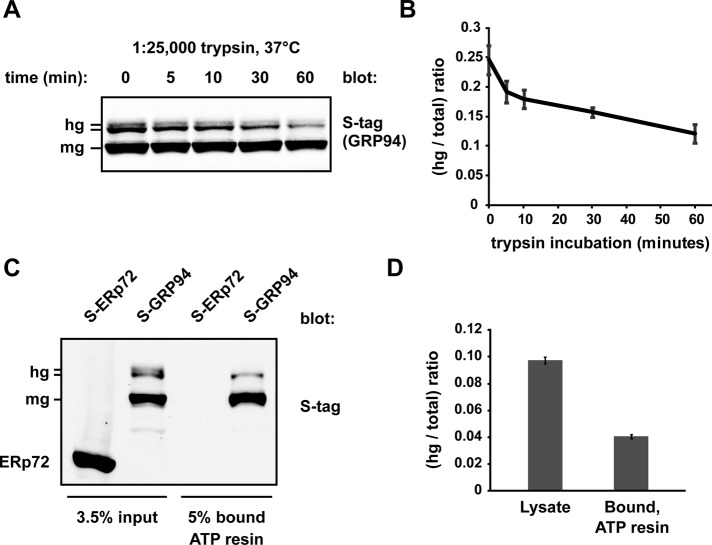
FIGURE 6.
hgGRP94 species exhibit altered conformation and lower ATP-binding activity. (A) S-GRP94–containing cell lysate was treated with dilute trypsin (1:25,000) for the indicated time course and then subjected to SDS–PAGE and immunoblotting with the anti–S-tag antibody. hg, hyperglycosylated S-GRP94; mg, monoglycosylated S-GRP94. (B) Quantification of three independent limited proteolysis experiments as in A. Bands corresponding to hgGRP94 were quantified as a fraction of total GRP94 and plotted over the 1-h time course. Means and SDs are plotted. (C) Affinity purification of S-ERp72 and S-GRP94 by γ-phosphate–linked ATP resin. Whole-cell lysate inputs and ATP-bound fractions were separated by SDS–PAGE and the resulting immunoblots probed with anti–S-tag. S-ERp72 was used as a control to ensure the ATP resin was specific for ATPases. (D) Quantification of three independent affinity purification experiments as in C; the hgGRP94 bands were quantified as a fraction of the total GRP94. Means and SDs are plotted.