Abstract
The earthworm is ideal for studying action potential conduction velocity in a classroom setting, as its simple linear anatomy allows easy axon length measurements and the worm's sparse coding allows single action potentials to be easily identified. The earthworm has two giant fiber systems (lateral and medial) with different conduction velocities that can be easily measured by manipulating electrode placement and the tactile stimulus. Here, we present a portable and robust experimental setup that allows students to perform conduction velocity measurements within a 30-min to 1-h laboratory session. Our improvement over this well-known preparation is the combination of behaviorally relevant tactile stimuli (avoiding electrical stimulation) with the invention of minimal, low-cost, and portable equipment. We tested these experiments during workshops in both a high school and college classroom environment and found positive learning outcomes when we compared pre- and posttests taken by the students.
Keywords: conduction velocity, giant fiber, cable theory, electrophysiology, earthworm, SpikerBox, LS1.D-information processing
students doing these experiments will learn how to measure the conduction velocity of neural action potentials using an earthworm preparation and will get an introduction to a basic electrophysiology laboratory setup using simple amplifiers and laptop computers to amplify and record neural data. Students will also learn how the earthworm's lateral and medial giant nerve fibers transmit different sensory information from different parts of the worm (allowing the escape withdrawal reflex in awake, behaving worms). Advanced students can also learn 1) axonal cable theory and 2) statistical hypothesis testing.
Background
Learning neurophysiology, in particular the electrical propagation of action potentials, can be challenging for students. Having a hands-on electrophysiology component to complement neuroscience lectures and laboratories makes the lessons both more compelling and increases learning (20). Moreover, evidence suggests that an active, inquiry-based learning pedagogy for science learning at the high school and university level (12, 21, 24) improves student comprehension and retention of scientific concepts, causing a sustained level of interest in pursuing science-, technology-, engineering-, and medicine-based careers.
Teaching inquiry-based neuroscience can be difficult for the biology/physiology teacher, as the combination of live animals paired with concepts of electrical signaling and amplification can be complicated to implement under the time and budgetary constraints of the high school/small college classroom. Some universities have had notable success in building neuroscience training laboratories, such as Cornell University with its CRAWDAD program (19, 25) and the University of Minnesota BrainU high school teacher training program (9).
Neurophysiology instructional laboratories are usually expensive to maintain, requiring preparation by knowledgeable faculty members with extensive experience in bioinstrumentation and/or electrical engineering. We (6, 20) have previously developed experiments that introduce students to the concept of action potentials and rate coding using cockroaches and crickets; here, we developed experiments using earthworms on the more challenging concept of conduction velocity.
We build on the work of other groups that have used the earthworm as a teaching platform (7, 17); our focus was on the portability and minimization of equipment. Typically, action potentials are evoked in anesthetized worms with an electrical stimulator and tabletop amplifiers; such “rigs” can often be intimidating for first-time users and may turn students away from electrophysiology. To combat this, we designed a simple, handheld two-channel amplifier (the two-channel SpikerBox) that, coupled with tactile stimulation in lieu of electrical stimulation, makes for a compelling conduction velocity experiment in the earthworm Lumbricus terrestris (colloquially called the “nightcrawler”).
The earthworm possesses one median giant fiber (MGF) and two lateral giant fibers (LGFs), both of which run the length of the worm and are located in the worm's ventral nerve cord (Fig. 1). Through careful experiments with nerve cuts, it has been shown that the LGF transmits sensory information from the posterior end and the MGF transmits sensory information from the anterior end of the worm (31), ultimately resulting in the “escape withdrawal reflex” of succinct muscular contractions (8, 26, 28–30). The diameter of the giant fibers is ∼0.05 mm for the LGF and 0.07 mm for the MGF. The two LGFs are fused together via frequent interconnects and thus are considered one functional giant axon (5, 13).
Fig. 1.
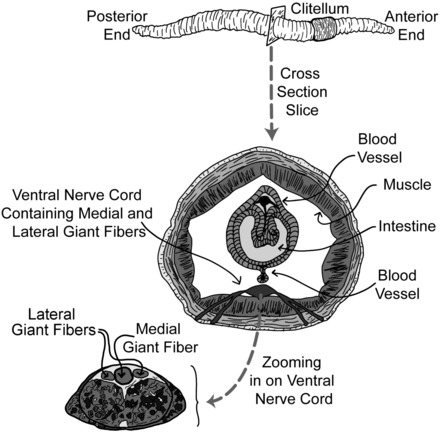
General earthworm anatomy (top) with a cross-section (bottom) zooming in on the ventral nerve cord with a view of the lateral giant fibers (LGFs) and medial giant fiber (MGF).
In this report, we describe our experiments designed with the intent to be easily followed by a student or teacher. We also discuss the potential troubleshooting and pitfalls that may occur while doing the experiments.
Learning Objectives
Using the National Research Council's Next Generation Science Standards (22) as a guideline, these lessons fall under Life Sciences 1–From Molecules to Organisms: Structures and Processes, subgroup LS1.D–Information Processing. Our experiments teach how an organism (the earthworm) detects and processes information about a tactile stimulus (a head or tail tap) by converting the tactile stimulus into an electrical signal (action potentials of neurons) and then propagating these electrical information signals throughout the animal's body, all of which can be observed via the measurement of neural conduction velocity. Students can use this knowledge to observe how an unanesthetized worm responds to a light touch on its head or tail with the escape withdrawal reflex: a touch (the signal detection) causes neuronal spiking (information processing), which sends a signal to the muscles to contract (the behavior).
Students will also gain general process knowledge, such as how to observe, collect, and analyze physiology data. Enthusiastic students can also learn 1) statistical hypothesis testing between two populations of data and 2) novel experiment design (see the discussion for some suggestions).
Activity Level
This activity is suitable for high school students and above who have a basic conceptual understanding of what an axon and an action potential are. Since electrophysiology is novel for many students and the methods are sensitive to electromagnetic noise that can easily confuse and frustrate students, close observation and help by the teacher during experiments (especially during data collection for the first worm) is essential (see Troubleshooting below). A teacher may find doing a quick demonstration of one conduction velocity reading at the beginning of class to be helpful.
Prerequisite Student Knowledge or Skills
Before doing this activity, students should have a basic understanding of neuron and earthworm anatomy. They should also be introduced to the concepts of action potentials and that they progress down an axon with a conduction velocity that can be measured. These can be discussed with the class during a 25- to 50-min lecture before the experiment session.
Students should know how to use an audio recording program (such as Audacity, as described below) to record earthworm action potentials (“spikes”) and to subsequently measure the time difference between spikes.
Time Required
An instructor experienced with this preparation can perform it in one worm in ∼10–20 min during a lecture demonstration. For a student laboratory, a student can perform the experiment with supervision in ∼45 min. The creation of a data set of five to seven worms to allow for statistical hypothesis testing of the different speeds will take a student ∼3–4 h.
METHODS
Equipment and Supplies
Anesthetics.
All experiments in this report were performed on worms under a 10% by volume ethanol anesthetic solution. The 10% ethanol solution was prepared by mixing 30 ml of tap water with 10 ml of 80 proof (40% ethanol) vodka. We placed the earthworms in the alcohol anesthetic for ∼5 min, briefly rinsed them off in tap water, and then began the experiments.
Carbonated water can also be used as an anesthetic if ethanol is not available. Carbonated water (60%) can be prepared by mixing 30 ml of sugar-free seltzer water (also called “club soda” or “sparkling water” at grocery stores) with 20 ml of tap water.
Anesthetic effectiveness can be determined by observing a lack of worm movement and a cessation of the escape withdrawal reflex. The escape withdrawal reflex can be observed by tapping the tail and head with a plastic probe. An alert worm will exhibit a shortening muscle contraction in response to this stimulus, but an anesthetized worm will not have this reflex. The typical time in the alcohol or carbonated water solution for sufficient anesthesia is ∼5 min.
After the anesthetic had taken effect, the worm was removed and placed in a container of tap water for several seconds to wash off the anesthetic. The effects of the anesthetic typically last 5–10 min. The worm was periodically kept moist during experiments with a wet cotton swab brushed along the worm. It is important to not leave the worms in the anesthetic solution excessively, as the worms will not produce action potentials and can also perish.
Equipment and software.
Figure 2 shows our experimental setup. Earthworms were placed on a balsa wood or styrofoam board with measurement marks drawn on the board. This board was then placed in a small Faraday cage with open ports to access the head and tail of the earthworm. The recording electrodes [electrode 1, electrode 2, and reference (sometimes also called “ground”)] inserted into the worm connected to our two-channel SpikerBox, which is a custom 880× gain two-stage amplifier using the AD623 instrumentation amplifier chip (Analog Devices, Norwood, MA) on the first stage and the TLC2272 operational amplifier chip (Texas Instruments, Dallas, TX) on the second stage (see Ref. 3 for the schematics). The first stage has a 2-GΩ input impedance and a gain of 4×, and the second stage has 220× gain and a band-pass filter from 300 to 1,300 Hz. The audio component of the amplifier uses a standard configuration of the LM386 audio chip (Texas Instruments). The output of the two-channel SpikerBox is a standard 3.5-mm (1/8 in) stereo audio jack that can then go into the line in of a laptop computer (preferably running on battery power to reduce any potential power line electrical noise). If the computer does not have a stereo line-in jack, a USB sound card can be used, such as the iMic (US$40, Griffin Technologies). USB sound cards can sometimes generate a 1-kHz ringing noise artifact, but this can often be grounded out by carefully positioning the earthworm in the Faraday cage.
Fig. 2.
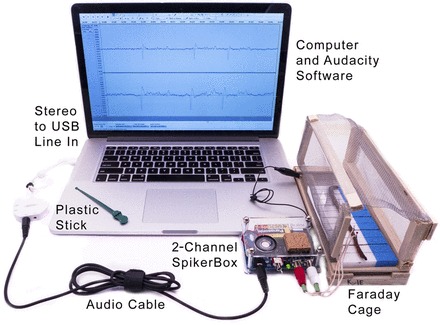
Experimental setup and equipment. We had a laptop with a two-channel USB sound card (iMic, Griffin Technology) and used Audacity, an open-source recording program. The electrodes were map pins soldered to speaker wire. We used a Faraday cage to reduce noise and had an earthworm on a styrofoam recording platform marked with 2.5-cm hashes. We grounded the SpikerBox to the Faraday cage using an alligator clip. The plastic stick or glass rod was our means of providing tactile stimuli to the earthworm. Some laptops have stereo input and do not need a two-channel USB converter.
To record the signals and measure the time difference between the two electrodes, we used the open-source audio processing program Audacity, which has versions available for all platforms (Mac, Windows, Linux, etc). In the preferences of Audacity, the input signal was set to “line in,” the hardware playthrough checkbox was enabled (so as to simultaneously hear the recordings as the signals are coming in), the number of channels was set to two (“stereo”), and the “record” red button was clicked to begin a recording session.
The earthworm preparation can be sensitive to noise, and a Faraday cage is essential to reduce spurious electromagnetic interference. A Faraday cage (such as the one shown in Fig. 1) can be easily assembled at low cost (less than US$10) using components from a local hardware store. Students can cut out an 8 × 16-in. rectangle of screen door metal mesh and five 8-in. lengths of wood strips (for example, 3/8-in. tall × 3/16-in. wide basswood). The wood strips can then be stapled to the mesh (with the first strip at one end of the of mesh, the second strip 5.5 in. away from the first strip, the third strip 2.5 in. away from the second strip, the fourth strip 5.5 in. from the third strip, and the fifth strip at the other end of the mesh). The mesh can then be folded into a box, and an alligator clip can be connected from any point on the metal mesh to the ground metal pad on the SpikerBox circuit board (or ground on any other system). Further details can found online (4). Enterprising students can also find that metal containers, such as antique lunchboxes, work as well.
Although we developed our own equipment and used an open-source software package for the experiments described here, equipment from other suppliers, such as from iWorx (two NA-100 amplifiers with the ETH-402 interface), AD Instruments (PowerLab 15T), Biopac (MP36R System), and Grass (two LP511 amplifiers), will all work as well.
Human or Animal Subjects
Human participants.
We held workshops in both a neuroscience class at a selective liberal arts college and a biology class at a suburban high school in Michigan. Our 2-h workshops were a mix of lectures and demonstrations that covered neural communication, conduction velocity, and cable theory. Students observed and assisted in live demos of the experiments listed in this report and then actively engaged in discussion about the theory and experiments.
Before and immediately after the workshops, on the same day, students were given multiple-choice tests to examine their knowledge of conduction velocity concepts. The pre- and posttests were exactly the same. The college survey had 13 questions that covered conduction velocity physiology and cable theory along with some mathematical questions dealing with time constant and length constant equations. The high school survey had eight questions and did not have the mathematical questions dealing with time constant and length constant equations. Of the 15 respondents at the college, 57% were women and 43% were men; 85% identified themselves as Caucasian, 7.5% as Asian, and 7.5% unreported; and the average age was 21.3 yr (SD: 0.63, range: 20–22 yr). Of 25 respondents at the high school, 52% were women and 48% were men; all but 1 respondent identified themselves as Caucasian (1 respondent was of mixed race); and the average age was 15.5 yr (SD: 0.5, range: 15–16 yr).
All survey data collection was performed with informed consent from teachers and students. Our testing policies were approved by the Institutional Review Board of Albion College.
Animal models.
The earthworm L. terrestris, commonly known as the Canadian nightcrawler, was our model of choice given its large size and ease of collection from local pet/sporting goods stores. Boxes of 12–18 worms typically cost US$3–4, and these worms can even be harvested in the field after a heavy rainfall.
Worms were stored in a refrigerator in a styrofoam container filled with soil, and we poured a small amount of water in the box every couple of weeks to keep the soil moist. The worms are very easy to care for; we have observed that they survive for ∼1–3 mo in the refrigerator.
The worm's clitellum can be used as an anatomic landmark since it is always closer to the anterior end (head) than the posterior end (anus; Fig. 1). Because the clitellum becomes more pronounced as the earthworm reaches sexual maturity, the experiments are best done on large, sexually mature adults. If the worm has not yet reached sexual maturity and the clitellum is hard to identify, close examination of the posterior end (anus) will show a flattening, whereas the anterior end (head) will be cylindrical and cone shaped.
Earthworms, as invertebrates, do not require institutional oversight or approval for experimentation in a classroom or research laboratory. However, we opened the experiments in the classrooms by stating the earthworms can recover from the experiment, discussing the use of anesthesia for humane treatment, mentioning the broader role of animals in physiology research, and pointing out an alternative noninvasive experiment (see Experiment 2 below).
Instructions
Experiment 1: comparing the speed of two fibers.
After the worm was anesthetized, we placed the worm dorsal side up [the dorsal side of worm is darker (brown) than the ventral side (gray)] on the wood/styrofoam platform and inserted the three electrodes into the worm slightly off its centerline so as to try to avoid piercing the intestine or ventral nerve cord. The metal pin electrodes traversed through the worm and into the wood/styrofoam base. Figure 3 shows the electrode positions. We placed the worm into the Faraday cage, and we grounded the Faraday cage to the SpikerBox using an alligator clip cable. Using a ruler or the hash marks drawn on the styrofoam board, we recorded the distance between electrode 1 and electrode 2 (where dLGF is the LGF electrode distance measurement and dMGF is the MGF electrode distance measurement).
Fig. 3.
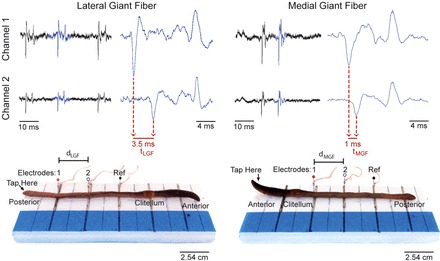
LGF and MGF action potentials and the corresponding electrode placement. Channel 1 and channel 2 are shown at a 10-ms timescale (left) and a zoomed-in 4-ms timescale (right). The LGF response was recorded by applying a mechanical stimulus to the posterior end, whereas the MGF response was recorded by applying a mechanical stimulus to the anterior end (see “tap here” arrow). Note the different electrode positions in both cases. To observe a time lag difference between channels 1 and 2, the waveforms must be zoomed in. Electrode 1 corresponds to channel 1, and electrode 2 corresponds to channel 2. dLGF, LGF electrode distance measurement; dMGF, MGF electrode distance measurement; Ref, reference electrode.
To first record from the LGF, we turned on the SpikerBox and started our Audacity recording. We then lightly tapped the very posterior end of the earthworm with a glass or plastic probe. In the seven worms, this mechanical stimulus typically evoked one to three spikes per tap (Fig. 3). We then turned off the SpikerBox but left Audacity running to create an easily recognizable flat line area that can be used as a separation marker between our LGF and MGF spikes, making data analysis easier. While the SpikerBox was off, we removed the worm from the Faraday cage and repositioned the electrodes in the anterior end of the worm to record from the MGF (Fig. 3). Again, we measured the distance between electrode 1 and electrode 2 using a ruler (dMGF). We placed the worm back into the Faraday cage and turned the SpikerBox back on. We then caused the spiking response in the MGF by tapping the anterior end of the worm (head) with the plastic or glass probe. After several spikes were recorded, we removed the electrodes from the worm and placed the worm back in its moist, soil-laden case in the refrigerator. Cleaning consisted of dumping out the anesthetic solution and wiping the electrodes and styrofoam with a wet cloth.
The Audacity file can then be saved in Audacity's format or exported as a .wav file to be analyzed in a program of choice (such as Matlab). To analyze the data after a recording in Audacity, we zoomed in on the evoked spikes until we were able to see time differences between the two channels (Fig. 3). Using the time markers on the Audacity window, we measured the time difference between the first large negative deflections of the spikes (where tLGF is the LGF time difference and tMGF is the MGF time difference). We then calculated the conduction velocity by dividing the ruler measurement by the time measurement, as follows:
where CVLGF and CVMGF are the conduction velocity measurements for the LGF and MGF, respectively.
For a good data set, evoked multiple spikes from the MGF and LGF can be collected and measured both in a single worm and across multiple worms (Fig. 4). This experiment takes ∼30–120 min to set up and complete depending on the number of worms used. Worms can recover from the experiment and be used again at a later date.
Fig. 4.
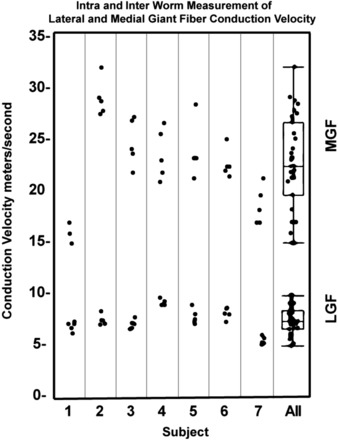
Intra- and interworm measurements of LGF and MGF conduction velocities. The two right box plots show the variance of the data for all seven worms. A paired t-test with an α-level of 0.05 gave P < 0.0001 between the LGF and MFG conduction velocities.
Experiment 2: invasive versus noninvasive recording.
Although the use of electrodes inserted in the earthworm keeps the earthworm firmly in place, some students may experience emotional discomfort inserting needles into the creature. Fortunately, it is possible to record spikes ex corporeal using a similar setup as that in experiment 1.
Instead of placing the electrodes into the worm, the electrodes were taped down to the wood or styrofoam recording platform. The metric placement and distance between the electrodes was maintained as described above. The worm was placed on the recording platform in the same way as in experiment 1, dorsal side up, but resting on top of the electrodes. Care must be taken to ensure that the worm's skin is resting well on the electrodes.
To test whether the amplitude of action potentials recorded inside versus outside the worm's body was different, we built a custom four-channel SpikerBox. Having four channels required the use of a US$199 four-channel USB Audio Mixer (Maya44, Leonberg, Germany). Audacity was again used to record the spikes. The earthworm was placed on two electrodes, and the two remaining electrodes were inserted into the worm as close as possible to the surface electrodes without physically touching and shorting (Fig. 5). The ground electrode was inserted a distance away and can be either inserted into the worm or rest below the worm.
Fig. 5.
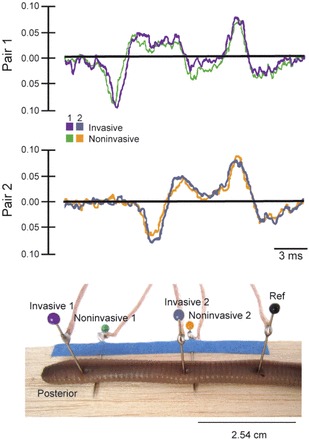
Invasive versus noninvasive electrode placement. Recording action potentials both invasively or noninvasively in the same worm showed little difference in amplitude and wave shape. The reference electrode (black) was inserted into the animal, but it can also be placed under the animal and functions as a reference similarly.
Troubleshooting
Sometimes electrode placement between the LGF and MGF can confuse students. The easiest way to resolve this is to take out the electrodes, turn the worm 180° around, and then simply place the electrodes back in the worm. The MGF was always faster than the LGF in our experiments, so students can in fact use their conduction velocity measurements to confirm the anatomy of the worm (for example, if the clitellum is hard to identify in a young worm).
One limitation of our experiments is when a nonspiking worm preparation occurs. If the worm is in an electrically noisy environment or is overanesthetized, spikes often cannot be either evoked or discriminated. As the amplitude of the spikes from the earthworm can be small due to their myelination (14), they can be easily buried in electrical noise. In addition, the very lack of many spikes, which makes the experiment compelling for conduction velocity measurements, also simultaneously makes the experiment hard to debug (is it my equipment, or is it the worm, that is not working?). We have found that starting the experiment using only lightly anesthetized worms can help students get used to the preparation and what the spikes “look and sound like” before experimenting on more deeply anesthetized preparations, where spikes may sometimes not occur. A lightly anesthetized worm will be moving slightly, and muscle electrical activity will be present in the recordings, occasionally masking the spikes, but a student can much more easily hear and identify the spikes before moving on to a more anesthetized worm to achieve a more accurate measurement of conduction velocity.
Another limitation of our experiments is that the two-channel measurement requires the use of a computer/laptop with stereo input instead of more portable mobile devices, such as tablets or smartphones. The use of the computer [given our previous work using only mobile devices (20)] makes the conduction velocity experiments slightly more cumbersome in cases where tablespace may be limited. We are researching wireless solutions for our amplifiers so that the two channels can be recorded and displayed on a mobile device.
Finally, the biggest issue that students may face is electromagnetic noise interfering with the recordings and drowning out the earthworm spikes. The use of a Faraday cage is critical in reducing such noise, but occasionally noise will still dominate, especially on the upper floors of tall buildings near radio or cellular transmission towers. In these situations, laptops should run on battery power alone and the SpikerBox and earthworm should both be placed in the Faraday cage. All wires should be folded together as much as possible to avoid creating antenna loops. Such intense noise is rare and is typically only observed near transmission equipment; this can be solved by changing the room where the experiment is being held.
Moreover, if the electrodes are not firmly in contact with the worm, the recordings may reveal popping and chirping when the worm is tapped due to the disruption of the electrode-worm interface. It is critical to ensure that 1) the electrodes are in contact with the worm (with the worm either laying on top of the electrodes or the electrodes inserted into the worm), 2) the audio cable from the two-channel SpikerBox is fully plugged into the laptop, and 3) plastic or glass probes instead of metal objects are used to tap the worm. The use of a lightly anesthetized worm that is robustly spiking to head or tail taps will help debug noise issues. Spurious noise can also occur if a part of the worm is in contact with the metal mesh of the Faraday cage. No part of the worm should touch the metal mesh. Also, if excessive water is on the platform (styrofoam or wood) that the worm is resting on, and if such water leaks down the side of the platform and makes contact with the metal mesh of the Faraday cage, spurious noise will also result, which can be difficult to identify. We sometimes place the wooden or styrofoam platform on top of a piece of acrylic plastic inserted into the Faraday cage to avoid this.
When tapping the worm, care should be taken to not be too vigorous with the tapping, such that the head or tail “flops up and down” with each tap. Such movement of the worm can cause spurious noise transients that look like action potentials on the recording but are not (spurious transients will happen at the exact same time on both channels and are thus not biological).
Use of Other Worms
California red worms (Eisenia fetida) are commonly used in composting and are easier to raise in self-sustaining colonies than L. terrestris; we have had self-sustaining colonies of such red worms for 3 yr. We attempted to replicate the conduction velocity experiments in red worms, but results were mixed. If the worms are anesthetized such that they are no longer moving, we only occasionally could elicit spikes in the MGN and never in the LGN. To record spikes successfully from the LGN, we had to anesthetize the worm only very lightly, such that it was still moving substantially, making measurement difficult. We recommend using the smaller E. fetida worms only when L. terrestris is not available, as getting stable recordings is difficult and can really only be done by a determined student or experienced instructor.
Our experiments in keeping L. terrestris in large plastic bins to make our own self-sustaining colonies were usually not successful, with the worms dying within 1–2 mo. However, one of us (W. J. Wilson) has two colonies that have survived well for 6 mo in temperature-controlled environments; time will tell whether these worms reproduce and the colonies become self-sustaining. For all experiments in this work, we simply bought L. terrestris from bait supply stores.
Safety Considerations
The needles we insert into the worm are commonly available map pins; students should take normal precautions when handling the pins (always picking up by plastic ball end and not the sharp end). The anesthetic we prepare is 10% ethanol, which can be prepared by the teacher before the experiment to avoid any connotations with alcohol consumption. In schools that do not allow experiments that use ethanol as a reagent, carbonated water can be used as an alternative anesthetic.
RESULTS
Experiment 1: Comparing the Speed of Two Fibers
We recorded the conduction velocity from seven worms for the data in this report but have since replicated this result many times during classroom lectures. Figure 3 shows sample recordings and traces from an individual earthworm; Fig. 4 shows the compiled data from all seven worms. In each worm, we took five measurements from different spikes in both the LGF and MGF. Within each worm, the difference between the MGF and LGF was immediately apparent and statistically significant (P < 0.05 by t-test). Across all worms, the average speed of the LGF was 7.6 ± 1.2 m/s (mean ± SD) and the MGF was 22.8 ± 4.5 m/s (mean ± SD). The differences are large enough that even with a low number of samples this exercise can also serve as an introduction to basic t-tests and statistical hypothesis testing for undergraduate students (1). In the Supplemental Material, we have included a .wav file of the MGF and LGF recordings that students and instructors can use as a reference when attempting to replicate these experiments.1
Experiment 2: Invasive Versus Noninvasive Recording
Although the earthworm can often recover from the insertion of the electrodes, recording ex corporeal can ease any students' potential emotional discomfort. Incidentally, the earthworm was actually the first preparation where ex corporeal recording of action potentials was demonstrated (29). Figure 5 shows recordings and electrode placement from simultaneous invasive and noninvasive recordings. Notably, the amplitude and waveform of the invasive versus noninvasive neural recordings was similar. Recordings in which the earthworm was lying on the ground electrode or the ground electrode was inserted into the earthworm were not different (data not shown). The only disadvantage of this ex corporeal recording is that the worm has to be under deeper anesthesia (placed in the anesthetic solution 1–3 min longer than our recommended 4 min), as any slight movement of the worm over the electrodes causes the recordings to be unstable. With deeper anesthesia, the risk is higher that the worm will not generate spikes when mechanically stimulated. The instructor should do a quick demonstration to all the students before the individual laboratory sessions, so that the students can learn how to mechanically stimulate the worm and see what the spikes look like on the computer screen.
Misconceptions
The action potentials recorded in this study are extracellular, meaning that the waveforms will not look like the action potentials depicted in textbooks. The shape of an extracellular action potential depends on the conductivity and electric fields surrounding the nerve and are on the order of ∼1 mV, much less than the 100-mV range of an intracellularly recorded action potential. Intracellular recordings are technically more difficult; the first successful intracellular recordings were actually done by inserting a glass microelectrode into the 1-mm-wide giant axons of a squid (16).
It should be clearly stated to the students that they are not inserting electrodes in nerves and that they are viewing an action potential recorded outside the nerve. For a comparison of the difference between intracellular versus extracellular recording traces, we recommend Fig. 1 in Ref. 15 as well Ref. 2 for a primer on voltage measurements in neural tissue.
Due to the earthworm spikes traveling past the recording electrodes and then passing the ground electrode, the spikes recorded from both channels have different initiation times but identical termination times [see the electrophysiological traces shown in Fig. 3, where there is a time delay in spike initiation (first negative deflection) but not in spike termination (final positive deflection)]. This should be pointed out to the students that they can only use the time delay in spike initiation to accurately measure conduction velocity.
Even though the axons in the earthworm are relatively large (0.05 mm for the LGF and 0.07 mm for the MGF) and students are commonly taught that invertebrates have large diameter axons to increase their conduction velocity during escape behavior, students may be surprised to learn that the conduction velocities are actually quite slow even in the large axons. We measured the conduction velocity at 22.8 m/s for the 0.07-mm MGN, or equivalent to ∼50 miles/h. Even the large 1-mm-diameter unmyelinated giant axon of the squid only has a similar speed of ∼20 m/s (27). To give students some perspective, the myelinated α-motor neurons of mammals can reach 80 m/s, or 180 miles/h, and are only ∼20 μm in diameter (10, 11).
However, it should be pointed out that earthworm nerves are also myelinated (14); teachers can explain this is why the conduction velocity of the MGN in the worm is roughly the same speed as the unmyelinated squid axon, although the earthworm nerves are ∼10 times smaller (see the appendix for a mathematical description of cable theory). This raises the interesting question as to why earthworm conduction velocity is not as fast (or faster) than much smaller (20 μm) mammalian myelinated α-motor neurons, which can conduct at 80 m/s. We do not have a satisfactory answer for this, but it can lead to an interesting discussion with students.
Teachers can also point out that although it is a general rule of thumb that invertebrates have unmyelinated axons and vertebrates have myelinated axons, vertebrates have a mix of both unmyelinated and myelinated axons in their bodies and some invertebrates, such as annelid worms and certain species of shrimp, have myelinated axons as well (14).
Evaluation of Student Work
College.
Our survey consisted of 13 multiple-choice questions, and the knowledge score was computed as the sum of correct answers. There was a significant difference in pretest (mean: 5.6, SD: 0.34) and posttest (mean: 8.8, SD: 0.53) knowledge scores [t(9) = −4.4956, P = 0.0015], suggesting that students improved their knowledge of core concepts of conduction velocity with a 25% average increase in test scores. Students notably increased their knowledge on questions on earthworm anatomy and general conduction velocity theory but did not noticeably increase their correct responses to the more difficult questions of what changes in capacitance and resistance across the neuron membrane will do to time constants and length constants. This is somewhat expected, as the math behind conduction velocity can be difficult to grasp over a 2-h-long workshop for novices being exposed to it for the first time. We are now working on handouts to give to the students before or after the lectures so they can study cable theory in more detail.
High school.
The knowledge survey consisted of eight multiple-choice questions, and the knowledge score was computed as a sum of correct answers. There was a significant difference in pretest (mean: 2.95, SD: 0.21) from posttest (mean: 3.81, SD: 0.23) knowledge scores [t(21) = −3.3563, P = 0.0030], suggesting that students increased their knowledge of conduction velocity concepts with an 11% average increase in test scores. Again, when we examined correct versus incorrect responses, students increased their knowledge on questions relating to earthworm anatomy and general conduction velocity theory but did not noticeably increase their correct responses to questions relating to the nodes of Ranvier or sparse coding. Handouts given to students after the lecture for them to study will probably increase their understanding of these concepts.
Inquiry Applications
Comparison with other published data.
Our results are close to agreement with Kladt et al. (17), whose group measured conduction velocities on the order of 16.9 m/s for the MGF and 6.9 m/s for the LGF using electrical stimulation in chlorobutanol-anesthetized worms. Our experiments are not in agreement with Drewes et al. (8), who measured MGF speed at 32.2 m/s and LGF speed at 12.6 m/s using tactile stimulation. Drewes et al. importantly, recorded conduction velocity in unanesthetized worms moving around a circular track covered with electrodes. We are beginning to prototype equipment, similar to Drewes et al.'s experimental apparatus, that will allow us to compare conduction velocities in awake behaving worms versus anesthetized worms to determine if the anesthesia is slowing conduction velocities.
Drewes et al. noted in their awake behaving recordings that when only one spike was elicited by tactile stimulation, a muscle contractile response was never observed. When two spikes were elicited, a contraction sometimes occurred, and when three or more spikes were elicited, muscle contractions were “consistently observed.” This would be an interesting result for students to try to replicate if they would like to build their own circular track. Moreover, Drewes et al. found 20% increases in conduction velocities (“facilitation”) of the second spike when multiple spikes were elicited by tactile stimulation (8). MGF second spikes were an average of 39 m/s, and LGF second spikes were an average of 14 m/s.
Although there does not appear to be a difference between electrical stimulation and tactile stimulation conduction velocities (our data compared with Kladt et al.'s data), there is a key difference between the two methods. During electrical excitation, spikes from both the MGF and LGF spikes are elicited regardless of electrode position, but, in our experiments with tactile stimulation, only MGF spikes are elicited when the head is tapped and LGF spikes when the tail is tapped. This is due to the sensory receptors in the head and tail only connecting to the MGF and LGF, respectively. A student could combine electrical and tactile stimulation to compare these two types of stimuli.
Experiments with electrical stimulation in our setup were not successful; the stimulus artifact would cause our SpikerBox circuit to become unstable (swamped) with a recovery time of 10 ms, masking the elicited spikes. We are planning to develop amplifiers with blanking to allow electrical stimulation experiments.
Wider Educational Applications
The experiments presented here can be used for a wide range of high school and undergraduate physiology classes. The main experiment, in which students examine the different conduction velocities of two different nerve systems in the earthworm, can be used as a basic teaching tool for action potential propagation and cable theory. The instructor can discuss how axonal diameter and myelination have specific electrical effects on how the spike travels down the axon (the MGF has a larger diameter than the LGF and thus has a faster conduction velocity). With the addition of mathematical and electrical principles, this experiment can easily lead to modeling work on cable theory in interested students. The appendix is a brief primer on cable theory and its relationship to LGF and MGF conduction velocity. Students can also begin exploring histology techniques to get physical measurements of the diameters of the fibers in each worm. We have also recently found that placing a segment of the worm over a block of ice in between the two recording electrodes will lower the conduction velocities by ∼50% and that the conduction velocities return to normal when the worm is back at room temperature. A student could carefully plot the relationship between temperature and conduction velocity, similar to Kladt et al. (17).
Experiments students can try that we have not tested are as follows: 1) Ruston and Barlow (29) noted that motor responses in awake worms to tactile stimulation were much more robust in a dark environment rather than a light environment and 2) Bullock (5) noted that that conduction velocity decreased as the animal was harmlessly stretched longitudinally; this is supposedly due to the diameter of the nerve cord changing (getting thinner due to the stretching).
GRANTS
Support for this work was provided by National Institutes of Mental Health Small Business Innovation Research Grants 1-R43-MH-093334-01 and 2-R44-MH-093334-03: “Backyard Brains: Bringing Neurophysiology Into Secondary Schools.” Our education research testing on high school and college students was reviewed and approved through the Institutional Review Board of Albion College.
DISCLOSURES
K. M. Shannon, G. J. Gage, A. Jankovic, and T. C. Marzullo are employees of Backyard Brains. W. J. Wilson has occasionally received equipment for testing/review from Backyard Brains.
AUTHOR CONTRIBUTIONS
Author contributions: K.M.S., G.J.G., A.J., and T.C.M. conception and design of research; K.M.S., G.J.G., W.J.W., and T.C.M. performed experiments; K.M.S., A.J., and T.C.M. analyzed data; K.M.S., G.J.G., W.J.W., and T.C.M. interpreted results of experiments; K.M.S. and T.C.M. prepared figures; K.M.S. and T.C.M. drafted manuscript; K.M.S., G.J.G., A.J., W.J.W., and T.C.M. edited and revised manuscript; G.J.G. and T.C.M. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Cristina Mezuk for help with literature searches and Gina M. Bello for expert proofreading and editing assistance.
Appendix
A Primer on Cable Theory and Earthworm Conduction Velocity Measurements
Time and length constants.
Cable theory, along with the Hodgkin-Huxley model of ion channel kinetics, is one of the most celebrated biophysical modeling accomplishments in neuroscience. Cable theory was originally developed in the 1800s, when engineers were trying to understand signal transmission across long-distance telegraph lines. While cable theory is occasionally covered in undergraduate neuroscience lectures, it can seem fairly abstract. These earthworm experiments can help give context to cable theory. The most important values relating myelin, axon diameter, and conduction velocity are the length constant (λ) and time constant (τ).
λ can be determined as follows:
where rm is the resistance across the axonal membrane and ri is the internal axon resistance. rm is a measure of how “electrically leaky” the axonal membrane is. The higher rm is and the lower ri is, the higher λ will be. λ (sometimes called the “space constant”) is a measure of how far a voltage change at one point in an axon travels down the axon before it decays. The voltage decays according to the following relationship:
where x is the distance away from the voltage change. If λ is 1 mm, then that means that at 1 mm away from where the initial voltage change occurred, 37% of the voltage magnitude remains. At 2 mm away from the cell body in an axon, 14% of the magnitude remains; at 3 mm away, 5% remains.
τ can be determined as follows:
where cm is the capacitance across the neuron membrane.
τ is a similar exponential function but applies to time (t) as follows:
If current flows across a neuron due to an ion channel opening, it takes time for the neuron to fully “charge” and reach a new stable voltage. If the neuron has a time constant of 1 ms, that means if a current change is applied across a neuron, after 1 ms, 63% of the new voltage is reached, after 2 ms, 86% of the new voltage is reached, and after 3 ms, 95% of the new voltage is reached. The smaller rm and cm become, the lower the time constant is and the less amount of time is needed to change an axon's voltage.
An “ideal neuron” would have an infinitely high λ value and an infinitely low τ value. Thus, any voltage change anywhere in the neuron would instantly affect the voltage everywhere else in the neuron.
Relation to conduction velocity.
MYELIN.
While commonly taught as a purely vertebrate invention, myelin-like coverings are used in some invertebrate animals, such as annelids and certain species of prawn shrimp (see Ref. 18; for an extensive review, see see Ref. 14). Covering the neurons with myelin makes the inside and outside of the neural membrane farther apart from each other, reducing cm, but this covering also substantially increases rm. The result of this simultaneous reduction in cm and increase in rm is hypothesized to cause no net change in τ, although direct experimental evidence in the literature is lacking.
However, since myelin does increase rm, it has a dramatic effect on λ. The result is such that the relationship between myelin thickness and conduction velocity is linear. Doubling the myelin thickness doubles the conduction velocity, tripling the myelin thickness triples the conduction velocity, and so on.
Increasing λ reduces the number of times that the action potential needs to be regenerated by voltage-gated ion channels as it travels down the axon; the ion channels each take ∼1 ms to open in response to voltage changes, so the less often ion channels have to open as an action potential propagates down an axon, the faster the conduction velocity will be.
LARGER-DIAMETER AXONS.
The other way that axons can increase their conduction velocity is by increasing the diameter of the axon and thus increasing λ as well. rm and ri are dependent on the constants Rm and Ri, which are based on the composition of the neural membrane and axoplasm. rm depends on the circumference of the axon, whereas ri depends on the cross-sectional area of the axon, as follows:
By removal of the constants, the equation simplifies such that λ is proportional to the radius of the axon, as follows:
This square relationship of axon radius to λ means that the axon would have to increase its radius four times to have an increase in λ of two times (and, thus, a corresponding 2-fold increase in conduction velocity).
Thus, myelin scales much faster than increasing axon diameter due to the myelin thickness' linear relationship to conduction velocity as opposed to the axon diameter's square root relationship to conduction velocity.
Predictions in the earthworm.
The diameter of earthworm giant fibers has been measured at ∼0.05 mm for the LGF and 0.07 mm for the MGF (5, 13). As these fibers are myelinated, we would expect the MGF to be 1.4 times faster than the LGF (0.07/0.05 = 1.4). As we measured the LGF speed to be 7.6 m/s, we would predict the MGF to then be 10.6 m/s. However, we experimentally measured the MGF to be 22.8 m/s (3 times larger velocity vs. 1.4 times larger expected difference) and currently cannot explain this higher than predicted difference. We are beginning experiments to histologically reexamine the size of the two fibers.
Footnotes
Supplemental Material for this article is available at the Advances in Physiology Education website.
REFERENCES
- 1.Backyard Brains. Experiment: Debunking the P-Value With Statistics (online). https://www.backyardbrains.com/experiments/p-value [26 June 2013].
- 2.Backyard Brains. Experiment: Referencing Your Spikes (online). https://backyardbrains.com/experiments/referencing [13 September 2013].
- 3.Backyard Brains. 2-Channel SpikerBox Bundle (online). https://backyardbrains.com/products/twochannelspikerboxbundle [30 October 2013].
- 4.Backyard Brains. Experiment: the Faraday Cage (online). https://backyardbrains.com/experiments/faraday [22 October 2013].
- 5.Bullock TH. Functional organization of the giant fiber system of Lumbricus. J Neurophysiol 8: 55–71, 1945. [Google Scholar]
- 6.Dagda RK, Thalhauser RM, Dagda R, Marzullo TC, Gage GJ. Using crickets to introduce neurophysiology to early undergraduate students. J Undergrad Neurosci Educ 12: A66–A74, 2013. [PMC free article] [PubMed] [Google Scholar]
- 7.Drewes CD. Non-invasive recording of giant nerve fiber action potentials from freely moving oligochates. In: Tested Studies for Laboratory Teaching, edited by Karcher SJ. Chapel Hill, NC: Proceedings of the 20th Workshop/Conference of the Association for Biology Laboratory Education, 1999, vol. 20, chapt. 2, p. 45–62. [Google Scholar]
- 8.Drewes CD, Landa KB, McFall JL. Giant nerve fibre activity in intact, freely moving earthworms. J Exp Biol 72: 217–227, 1978. [DOI] [PubMed] [Google Scholar]
- 9.Dubinsky J. Neuroscience education for prekindergarden-12 teachers. J Neurosci 30: 8057–8060, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gasser HS. The classification of nerve fibers. Ohio J Sci 41: 145–159, 1941. [Google Scholar]
- 11.Gasser HS, Graham HT. Potentials produced in the spinal cord by stimulation of dorsal roots. Am J Physiol 103: 303–320, 1933. [Google Scholar]
- 12.Geier R, Blumenfeld PC, Marx RW, Krajcik JS, Fishman B, Soloway E, Clay-Chambers J. Standardized test outcomes for students engaged in inquiry-based science curricula in the context of urban reform. J Res Sci Teach 45: 922–939, 2008. [Google Scholar]
- 13.Günther J. Overlapping sensory fields of the giant fiber systems in the earthworm. Naturwissenschaften 60: 521–522, 1973. [Google Scholar]
- 14.Hartline DK, Colman DR. Rapid conduction and the evolution of giant axons and myelinated fibers. Curr Biol 17: R29–R35, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Henze DA, Borhegyi Z, Csicsvari J, Mamiya A, Harris KD, Buzsáki G. Intracellular features predicted by extracellular recordings in the hippocampus in vivo. J Neurophysiol 84: 390–400, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Hodgkin AL, Huxley AF. Action potentials recorded from inside a nerve fiber. Nature 144: 710–711, 1939. [Google Scholar]
- 17.Kladt N, Hanslik U, Heinzel H. Teaching basic neurophysiology using intact earthworm. J Undergrad Neurosci Educ 9: A20–A35, 2010. [PMC free article] [PubMed] [Google Scholar]
- 18.Kusano K. Electrical activity and structural correlates of giant nerve fibers in kuruma shrimp (Panaeus japonicus). J Cell Physiol 68: 361–384, 1966. [Google Scholar]
- 19.Land BR, Wyttenbach RA, Johnson BR. Tools for physiology labs: an inexpensive high-performance amplifier and electrode for extracellular recording. J Neurosci Methods 106: 47–55, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Marzullo TC, Gage GJ. The SpikerBox: a low cost, open-source bioamplifier for increasing public participation in neuroscience inquiry. PLos One 7: e30837, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minner DD, Levy AJ, Century J. Inquiry-based science instruction–what is it and does it matter? Results from a research synthesis years 1984 to 2002. J Res Sci Teach 47: 474–496, 2010. [Google Scholar]
- 22.National Research Council. A Framework for K–12 Science Education. Washington, DC: National Academies, 2012. [Google Scholar]
- 23.Nicol JC. The giant axons of annelids. Q Rev Biol 23: 291–323, 1948. [DOI] [PubMed] [Google Scholar]
- 24.Prince MJ, Felder RM. Inductive teaching and learning methods: definitions, comparisons, and research bases. J Eng Educ 95: 123–138, 2006. [Google Scholar]
- 25.Pulver SR, Hornstein NJ, Land BL, Johnson BR. Optogenetics in the teaching laboratory: using channelrhodopsin-2 to study the neural basis of behavior and synaptic physiology in Drosophila. Adv Physiol Educ 35: 82–91, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts MB. The giant fibre reflex of the earthworm, Lumbricus terrestris L. I. The rapid response. J Exp Biol 39: 219–227, 1962. [DOI] [PubMed] [Google Scholar]
- 27.Rosenthal JJ, Bezanilla F. Seasonal variation in conduction velocity of action potentials in squid giant axons. Biol Bull 199: 135–143, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Ruston WA. Reflex conduction in the giant fibers of the earthworm. Proc R Soc 133: 109–120, 1956. [DOI] [PubMed] [Google Scholar]
- 29.Ruston WA, Barlow HB. Single-fibre response from an intact animal. Nature 152: 597–598, 1943. [Google Scholar]
- 30.Stough HB. Giant nerve fibers of the earthworm. J Comp Neurol 40: 409–463, 1926. [Google Scholar]
- 31.Stough HB. Polarization of the giant nerve fibers of the earthworm. J Comp Neurol 50: 217–229, 1930. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


