Figure 1.
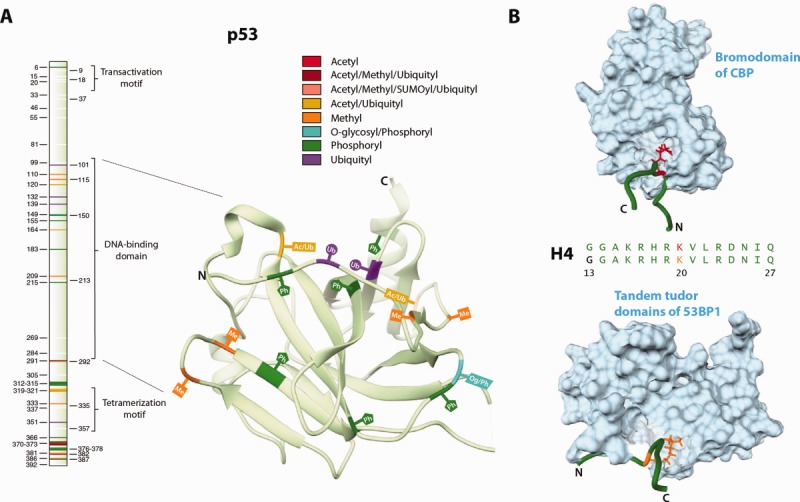
Examples of the three modes of concerted PTM regulation. (A) The primary sequence of the p53 tumor suppressor protein along with the structure of its DNA-binding domain (ID: 2YBG). The different PTM sites are highlighted and labeled in different colors. Sequential ubiquitylation of lysines in the DNA-binding domain and the C-terminus has been shown to regulate the nuclear export of p53.85 Additionally, relationships between different but overlapping clusters of N-terminal phosphorylation sites have been thought to check untimely p53 activation and enable signal integration and amplification over multiple stress pathways.86 A recent study identified 150 novel modifications and suggested that by virtue of the unusually high number of PTM sites, the combinatorial regulation of p53 is far more complex than previously thought.87 In this study, we refer to such proteins as being enriched in one or more types of PTM sites. (B) Acetylation and dimethylation of Lys20 in histone H4 results in recognition by the transcriptional coactivator CBP (ID: 2RNY, model 6) and the DNA-damage response protein 53BP1 (ID: 2LVM, model 10), respectively. Only the most informative NMR models as calculated by Olderado88 are shown here. A slight difference in structure can be seen, with the acetylated form having a larger bend than the dimethylated form. Each of these interactions results in a different functional outcome. The binding of CBP has a strong affinity in vitro89 and is speculated to increase the acetylation of H3 and H4 histones, which is generally associated with transcription activation.90,91 Unlike the recognition of the acetylation mark, the interaction between 53BP1 and dimethylated form occurs only in a specific cellular context and is important for the promotion of nonhomologous end-joining repair in response to DNA damage.92 Recently, a mass spectrometry-based study of in vivo histone acetylation dynamics reported that a sharp reduction in acetylation at Lys20 was due to increased methylation.93 For simplicity, we refer to such sites of competition (with more than one observed modification) as shared PTM sites, those sites with only one observed modification as single-PTM sites and those with no observed modifications as non-PTM sites.
