Figure 4.
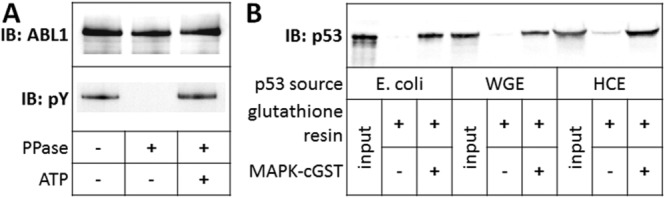
Functional assays of selected purified proteins. (A) Auto-phosphorylation of purified, dephosphorylated ABL1. ABL1 kinase was prepared by expression in HCE as an N-terminal HaloTag fusion protein, binding to resin, dephosphorylation and cleavage from the resin with TEV protease. Purified protein was then incubated with and without ATP. Phosphotyrosine signal was detected by immunoblotting with antiphosphotyrosine. (B) Interaction of purified p53 with MAPK1. The MAPK1-p53 interaction was queried by incubating HCE-produced MAPK1-cGST with p53 purified from E. coli, WGE, and HCE. The MAPK1-cGST and p53 mixtures were bound onto glutathione resin, washed, and boiled in SDS-PAGE sample buffer. Glutathione resin was incubated with p53 alone as a negative control. Immunoblotting was performed to detect p53 signal.
