Fig. 2.
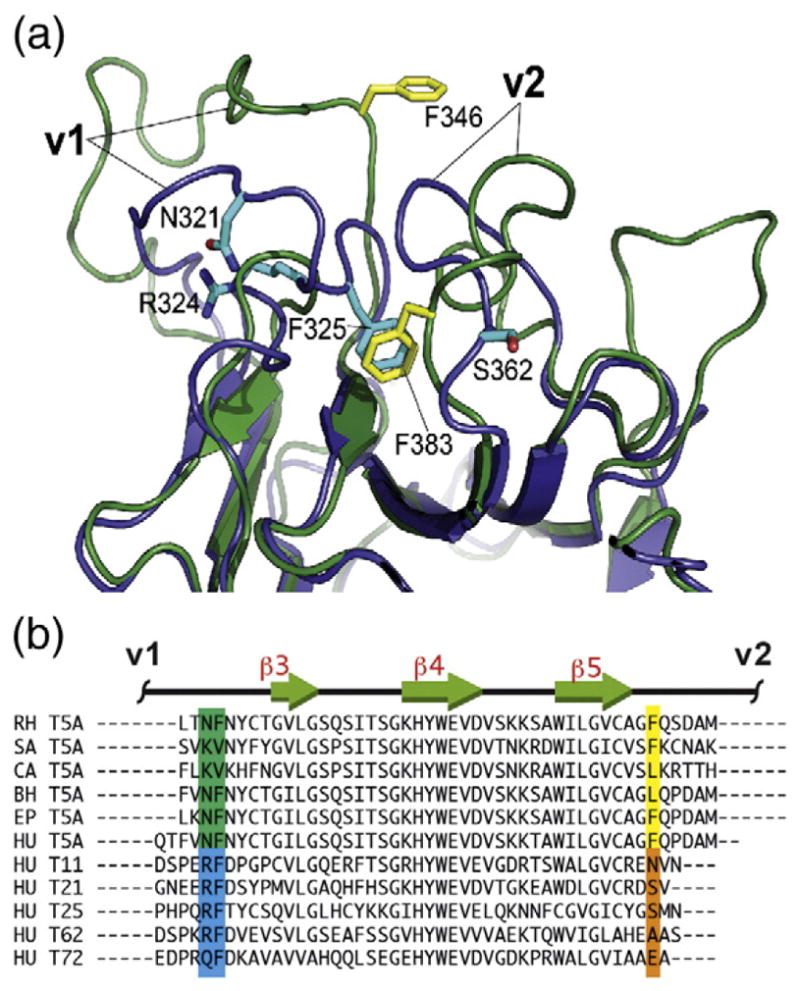
v1 mobility and the associated primary sequence patterns. (a) Superposition of one of the TRIM5 SPRY structures from the NMR ensemble (green, yellow side chains; PDB code: 2LM3) and the TRIM21 SPRY crystal structure (blue, cyan side chains; PDB code: 2IWG). F325 anchors the v1 segment to the protein core in the TRIM21 structure, whereas in the TRIM5 SPRY, the analogous pocket is occupied by the v2 residue F383. (b) Virtually all of the primate TRIM5 variants have an aromatic or large hydrophobic residue in the position corresponding to F383 of the rhesus TRIM5 (highlighted yellow). In contrast, most of the other SPRY-containing TRIM proteins in the human genome contain a small or polar/charged residue in this position (highlighted orange), which correlates with the occurrence of an RF motif in v1 (highlighted blue). Abbreviations: RH, rhesus monkey, Macaca mulatta; SA, white-lipped tamarin, Saguinus labiatus; CA, white-fronted capuchin, Cebus albifrons; BH, hoolock gibbon, Bunopithecus hoolock; EP, patas monkey, Erythrocebus patas; HU, Homo sapiens.
