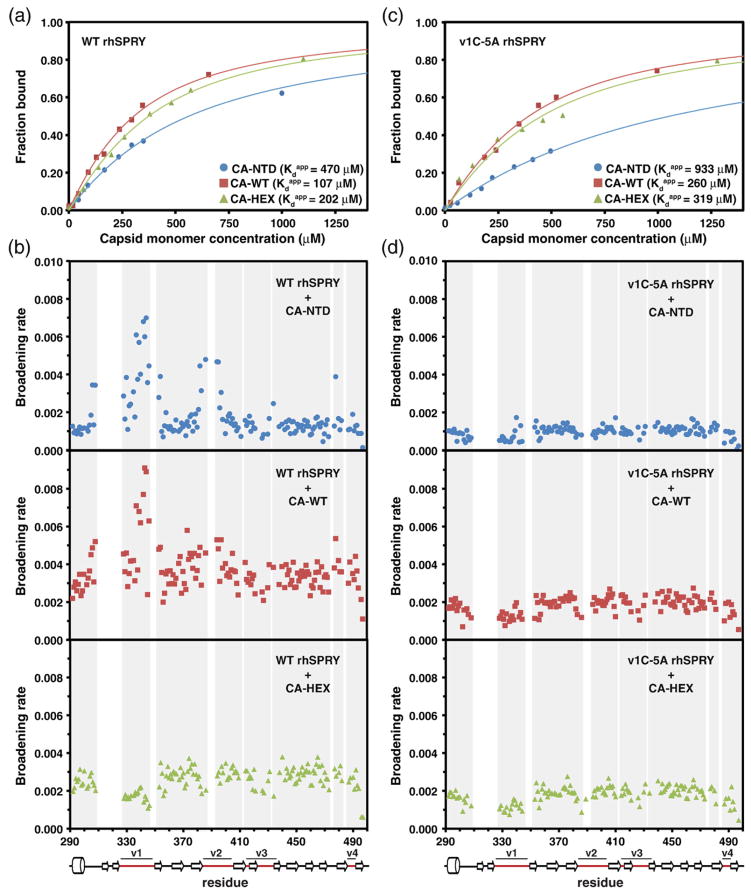
Fig. 5.
NMR titrations of the 15N-labeled SPRY. The wild-type rhesus SPRY construct (a and b) and the v1C-5A mutant SPRY (c and d) were titrated with three different capsid constructs: CA-NTD, CA-WT, and CA-HEX. Capsid concentration is given as the concentration of the capsid monomer for all titrations. The intensity attenuation upon addition of increasing amounts of capsid was converted to fraction bound as described in Materials and Methods. (a and c) The binding curves and the derived apparent dissociation constructs determined in the titrations of the WT SPRY (a) and v1C-5A SPRY (c) with the three capsid constructs. (b and d) The broadening rates of the backbone amide NMR signals plotted for all assigned SPRY residues. The enhanced broadening of the signals in the loops v1 and v2 is apparent when the WT SPRY is titrated with the CA-NTD and CA-WT construct, whereas it is not observed in the CA-HEX titration. The enhanced v1/v2 broadening is also not observed in the titrations performed with the v1C-5A SPRY mutant.