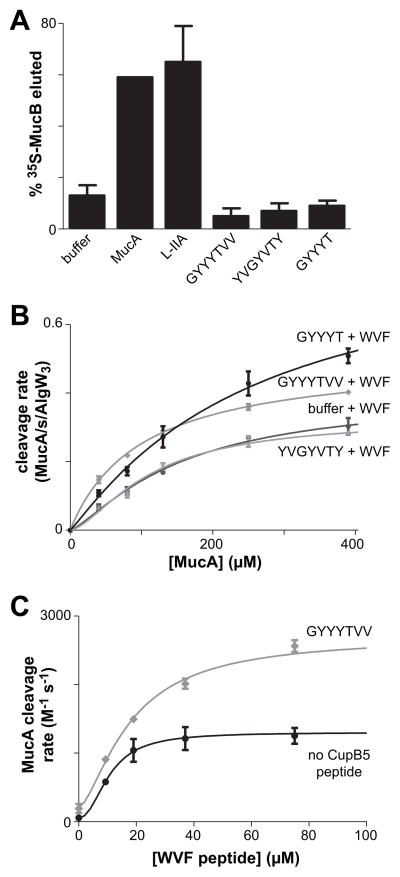
Fig. 5. CupB5 peptides do not compete with MucB for MucA binding but stimulate WVF-activated AlgW cleavage of MucA.
(A) Release of 35S-MucB from MucA-agarose following incubation with buffer, MucA (155 μM), L-IIA (20 mg/mL), or CupB5 peptides (~300 μM). For the CupB5 peptides, values are averages ± SEM (n=3). For L-IIA, the value is the average ± SEM (n=2). (B) Rates of cleavage by AlgW (0.5 μM trimer) and WVF peptide (150 μM) were measured at different concentrations of 35S-MucA in the presence or absence of CupB5 peptides (300 μM). Values are averages ± SEM (n=2) and were fit to the Hill form of the Michaelis-Menten equation, rate = Vmax/(1 + (KM/[substrate])h). (C) Rates of cleavage of 35S-MucA (50 μM) by AlgW (0.5 μM trimer) were assayed at difference concentrations of WVF peptide in the presence or absence of CupB5 peptides (300 μM). Values are averages ± SEM (n=2) and were fit to the equation rate = basal + Vmax/(1 + (Kact/[peptide])h). Without CupB5 peptide, h was 2.2 ± 1, Kact was 11 ± 2 μM, and Vmax was 1250 ± 150 M−1 s−1. With GYYYTVV peptide, h was 1.5 ± 0.8, Kact was 18 ± 7 μM, and Vmax was 2490 ± 490 M−1 s−1.