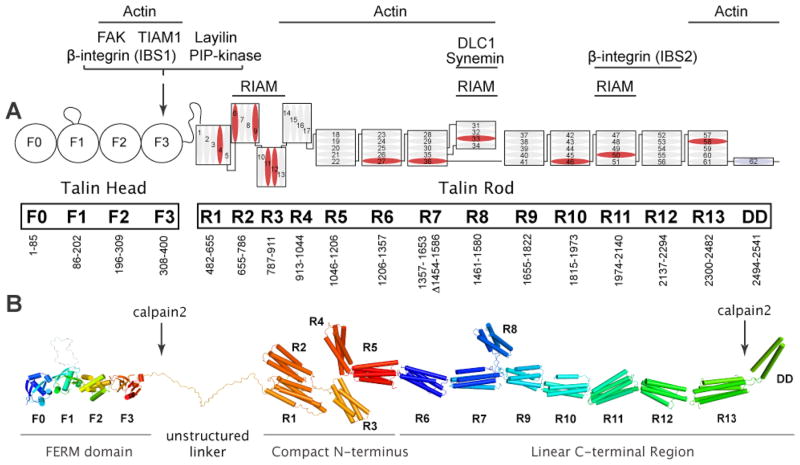
FIG 1. Domain organisation and structural model of full-length talin.
A: The domain organisation of talin135. The N-terminal talin head, which is comprised of an atypical talin FERM domain containing F0, F1, F2, and F3 domains, is joined by an unstructured linker of ~80 residues to the flexible talin rod. The rod is made up of 62 α-helices (numbered blue cylinders) that are organised into thirteen 4- or 5-helix bundles (R1–R13), with a single helical dimerisation domain (DD) at the C-terminus. Domain boundaries and the interaction sites for talin-binding proteins are indicated (IBS; integrin binding site). Helices that bind vinculin are in blue. Talin2 is predicted to have the same domain structure. B: Structural model of talin assembled from the crystal and NMR structures of the various domains. The position of the calpain2 cleavage sites are indicated. The R1 and R2 domains interact via an extensive hydrophobic interface34, and a long common helix joins R11 and R1230 (not shown). Otherwise, helical bundles are joined by short linkers (not shown since their structures were not determined). Because the N- and C-termini of the three 4-helix bundles (R2R3R4) are positioned at the same end of the bundle, this region will be more compact than the long succession of 5-helix bundles linked via their N- and C-termini.