Abstract
Objective
To determine the long-term effects of the cough stimulation system.
Design
Nonrandomized clinical trial of subjects using the study device well beyond the period of close follow-up.
Setting
Use of the study device in the home setting.
Participants
Subjects (N = 10) implanted with the device for a minimum of 2 years (mean 4.6 ± 0.6 years).
Interventions
Application of daily stimulation.
Outcome measures
Airway pressure generation and other clinical assessments including ease in raising secretions, life quality, caregiver support, and incidence of respiratory tract infections were measured at 1 year and mean 4.6 years after implantation.
Results
Each subject continued to use the device on a regular basis. During SCS, mean maximum airway pressures were 103.1 ± 20.4 and 107.7 ± 23.0 cmH2O at the 1-year and mean 4.6-year follow-up points, respectively (P < 0.05 compared with pre-implant and not significantly different (NS) compared with 1-year follow-up). Benchmarks related to ease in raising secretions and improvements in life quality related to respiratory care were maintained at the mean 4.6 year follow-up. The need for trained caregivers to provide other means of secretion management remained significantly below the pre-implant values (P < 0.05). The incidence of acute respiratory tract infections remained low at 0.2 ± 0.1 events/year, which is significantly below the pre-implant value of 1.4 ± 0.3 events/year (P < 0.05).
Conclusion
Subjects continued to use the system on a long-term basis beyond the period of close follow-up and to continued derive significant clinical benefits.
Keywords: Cough, Expiratory muscles, Spinal cord stimulation
Introduction
In a recent study, we demonstrated that lower thoracic spinal cord stimulation (SCS) produces near maximal activation of the expiratory muscles resulting in the generation of high peak airflow rates and expiratory airway pressures, characteristic of a normal cough.1,2 Importantly, restoration of a cough via SCS (cough stimulation system) proved to be a safe and effective method of airway clearance management resulting in a positive clinical impact in subjects with spinal cord injury (SCI). Use of this device resulted in significant reductions in the incidence of acute respiratory tract infections and need for caregiver support and improved life quality related to secretion management.1,3 The initial analysis of this investigation, however, was relatively short in duration involving close clinical follow-up and data analysis for the first 52 weeks following device implantation.
As with most investigations of this type, our study design required close monitoring of subjects and frequent scheduled interactions with the research staff to obtain physiological measurements and other clinical assessments. Close follow-up, however, may have imparted additional motivating factors to continue use of the device and satisfy the clinical goals of the study. It is conceivable that these factors may have exaggerated the actual benefit of this new therapeutic modality.
One test of the ultimate clinical utility of any experimental device is the degree to which observed clinical benefits are sustained. Further evidence is the desire of subjects to continue using the device as initially prescribed or on some other regular basis. The purpose of this study, therefore, was to re-assess the clinical parameters related to use of the cough stimulation system in spinal cord injured subjects who have had the implant for a minimum of 2 years, i.e. well beyond the period of close follow-up, in terms of their perceived clinical effects.
Methods
Background
This investigation was approved by the Institutional Review Board, the National Institute of Neurological Disorders and Stroke and the Food and Drug Administration. Before enrollment in the study, informed consent was sought and obtained from each subject. Each subject had suffered from a traumatic SCI, had secondary significant paresis of the expiratory muscles and had difficulty mobilizing secretions. Expiratory muscle weakness was confirmed by measurements of maximum expiratory pressures (≤40% predicted), measured at total lung capacity (TLC). No subjects had significant lung, cardiac or brain disease.
Each subject had undergone implantation of the cough stimulation system.1,2 Briefly, this procedure involved the midline placement of disc electrodes (4 mm) (Freehand Epimysial Electrode; NeuroControl Corp., 8333 Rockside Road, Valley View, OH, 44125, USA or Ardiem Medical, Inc., 1125 Wayne Avenue, Indiana, PA, 15701, USA) at the T9, T11 and L1 levels via hemilaminotomy incisions. A ground electrode was placed near the thoracolumbar fascia. A radiofrequency receiver (Finetech Medical Ltd., 13 Tewin Court, Welwyn Garden City, Herfordshire, AL7 1AU, UK) was placed in a subcutaneous pocket over the anterior portion of the chest wall. The electrode wires were tunneled subcutaneously and connected to the receiver. Subjects were instructed to apply stimulation every 30 seconds for 5–10 minutes, two or three times per day. Subjects also used the device on an as needed basis for evacuation of secretions. This stimulus paradigm was selected to initially recondition and then maintain the strength of the expiratory muscles and to provide a means of airway clearance.
The initial cohort of study participants included 17 subjects implanted with the device; five subjects died for reasons unrelated to participation in the study. Subjects were closely followed at monthly intervals for the first 6 months and subsequently at 3-month intervals for the next 6 months. Physiological assessments of expiratory muscle function via evaluation of maximal expiratory pressures and other clinical parameters of cough efficacy were conducted via a series of questionnaires (see below). Clinical assessments were obtained at study enrollment and at 28, 40, and 52 weeks after implantation of the cough stimulation system during face-to-face encounters. In the second year, subjects were followed with only brief bi-annual visits. Subsequently, subjects were followed with quarterly phone calls to assess their status.
Study design
This study involves the evaluation of those subjects who have had the device implanted for a minimum of 2 years (N = 10) with a follow-up of (mean ± SEM) 4.6 ± 0.6 years (range: 2.2–7.2 years).
Physiological assessments
Expiratory airway pressure generation was assessed using a BIOPAC Data Acquisition and Analysis System with AcquKnowledge software, MP100 system with TSD 160 pressure transducer (Biopac Systems Inc., 42 Aero Camino, CA, USA). Measurements were made with use of a tight-fitting full face mask or through a tracheostomy tube, which was present in one subject. In the seated posture, expiratory airway pressure measurements were made under conditions of airway occlusion at TLC. Cheek pressure was maintained manually during these measurements. These measurements were made in 7 of the 10 subjects as 2 subjects had moved out of the local area and 1 subject died from causes unrelated to the study prior to these measurements being obtained.
Other clinical assessments
The same questionnaires as those provided during the initial year of the study were reviewed with each of the 10 subjects either face-to-face or by phone. These included open-ended questions regarding use of the stimulation system and suctioning/assisted cough and sputum index to characterize the need for secretion removal and severity of such episodes. Since effective cough may reduce stress, life quality assessments were also performed. These include increased times for social activities, pursuit of recreational interests, and sense of well-being. Life quality assessments addressed issues specifically related to breathing, cough, and suctioning.
The incidence of acute respiratory tract infections as defined by a change in the character, color, or amount of respiratory secretions and requiring antibiotic administration for a 2-year period prior to implantation of the cough stimulation system was recorded. The occurrence of respiratory tract infections was determined by history and corroborated by review of the medical records, when these were available. Since implantation of the cough stimulation system, the incidence of acute respiratory tract infections has been monitored continually for each subject.
The degree of caregiver support was determined as the number of times it was necessary for a caregiver to provide the subject with any form of assistive means of secretion clearance including suctioning, manually assisted cough or use of the insufflation-exsufflation device. Caregiver support was evaluated over a 2-week period prior to implantation of the cough stimulation system and continuously over the course of the initial year and again at the time of this study.
Data analysis
The data obtained at the 1-year time point were compared to the pre-implant data and the data obtained during the mean 4.6-year follow-up using non-parametric analog (Freidman Test) to the standard repeated-measures analysis of variance. Statistical significance was taken at P < 0.05. This alpha level was used as a correlation for inflated Type I error rates because of multiple comparisons. Results are reported as mean ± SE.
Results
The baseline demographic data of the 10 subjects included in this analysis are provided in Table 1. The interval between the date of injury and participation in this trial ranged between 1 and 34 years (8.7 ± 3.5 years). At the time of enrollment, vital capacity measurements ranged from 11 to 62% predicted value (mean 35.1 ± 4.8%). Each subject had marked respiratory muscle weakness as reflected in maximum expiratory pressure measurements, which ranged from 5 to 35% predicted (mean 12.2 ± 1.7%).
Table 1 .
Clinical data of the subjects
| Sex | Age (years) | Cause of injury | Level of injury | Elapsed time since injury (years) | Spontaneous vital capacity (L) (% predicted) | Maximal expiratory pressure (cmH2O) (% predicted) |
|---|---|---|---|---|---|---|
| M | 52 | MVA | C5/C6 | 7 | 2.0 (39%) | 22 (10%) |
| F | 28 | MVA | C3 | 22 | 0.4 (11%) | 16 (10%) |
| M | 28 | Sport | C4/C5 | 2 | 2.6 (45%) | 24 (10%) |
| M | 50 | Violence | C5/C6 | 12 | 2.4 (47%) | 24 (11%) |
| M | 23 | Diving | C4/C5 | 1 | 0.9 (17%) | 13 (5%) |
| M | 49 | Diving | C3/C4 | 34 | 1.7 (30%) | 17 (8%) |
| M | 21 | MVA | C4 | 2 | 2.2 (42%) | 28 (12%) |
| M | 51 | Fall | T5 | 3 | 3.0 (62%) | 59 (25%) |
| M | 34 | Diving | C3 | 1 | 1.4 (24%) | 45 (15%) |
| F | 20 | MVA | C3 | 3 | 1.3 (34%) | 35 (16%) |
F, female; M, male; MVA, motor vehicle accident.
At the mean 4.6-year follow-up point, each of the 10 subjects reported that they continued to use the cough stimulation system on a regular, usually daily basis. While not included in our data analysis, several subjects stated that they were also using it for sneezing and/or to clear their nasal passages.
Mean maximum expiratory airway pressure generation during spontaneous efforts pre-implantation and post-implantation during SCS at the 1-year follow-up period were 28.3 ± 4.8, and 103.1 ± 20.4 cmH2O, respectively (Fig. 1). Current airway pressure generation (4.6-year follow-up) during SCS was 107.7 ± 23.0 cmH2O. Both values obtained during SCS were significantly higher than spontaneous efforts (P < 0.05). Importantly, airway pressure generation achieved with SCS at year 1 was maintained at the mean 4.6-year follow-up point (NS compared to 1-year follow-up) (Fig. 1).
Figure 1 .

Airway pressure generation during spontaneous efforts pre-implant (left panel) and during SCS after 1 year (middle panel) and mean 4.6 years (right panel) of follow-up. See text for further explanation.
The mean results of the suctioning/assisted cough and sputum index are shown in Fig. 2. In each instance, significant improvement following implantation in each of the parameters (P < 0.05 compared with pre-implant values) was maintained at the mean 4.6-year follow-up and was not significantly different than the responses observed at the 1-year follow-up (NS compared to 1-year follow-up). In fact, the need for suctioning/assisted cough on a typical day fell to zero (Fig. 2A). The severity of cough episodes was maintained at very low levels (Fig. 2B), and the difficulty in raising secretions remained in the mild range (Fig. 2C). These finding were corroborated by the responses to the ease in raising secretions with the cough stimulation system compared to previous methods, in which subjects reported marked improvement (Fig. 2D). With the exception of one subject who occasionally used a suction machine once/week, each subject reported that they relied on the cough stimulation system as their only method of secretion management.
Figure 2 .
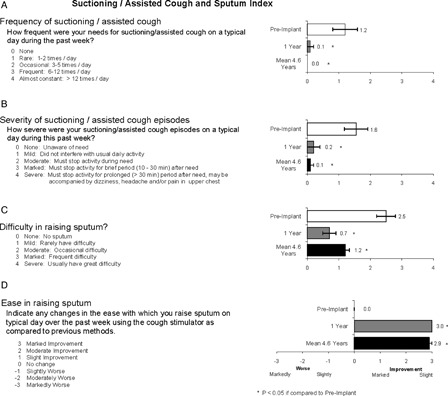
Subject responses to frequency of need for conventional means of secretion clearance, severity of such episodes, difficulty in raising secretions and change in ease in raising secretions using the cough stimulation system compared with previous methods. Compared to pre-implant, there were significant improvements in all parameters of secretion management at 1-year follow-up (P < 0.05). This improvement was maintained at the mean 4.6-year follow-up assessment (P < 0.05 compared with pre-implant; NS compared with 1-year follow-up).
Responses to questions related to subject life quality are provided in Fig. 3. Again, in each instance the improvements noted after 1 year were maintained at the mean 4.6-year follow-up (NS compared to 1-year follow-up). More specifically, these subjects reported that their overall physical condition or medical treatment had less interference with family life (question 1), the need for cough or need for assistance with managing airway secretions interfered less with daily activities (questions 3 and 5), there was less requirement for suctioning or other form of secretion removal (question 4), level of stress related to the need for coughing assistance was reduced (question 6), there was less embarrassment related to coughing or respiratory problem (question 7), and subjects experienced greater control of their breathing problems (question 8). There were also reductions in subjects' perceptions of financial difficulties (question 2). As with the 1-year follow-up time point, there were no significant improvements in overall health (question 9) or overall life quality (question 10) at the mean 4.6-year follow-up.
Figure 3 .

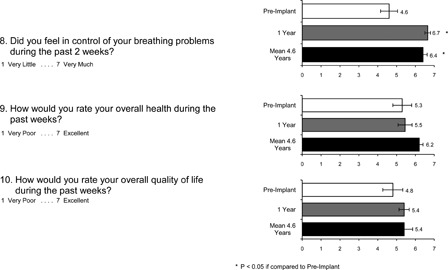
Subject responses to life quality assessment related to secretion management. There was improvement in most parameters at 1-year follow-up. These were maintained at the mean 4.6-year follow-up.
The requirement for caregiver support related to secretion management, as measured over a 2-week period, is shown in Fig. 4. At the 1-year endpoint, this parameter decreased dramatically from 10.4 ± 4.9 prior to device implant to 0.2 ± 0.1 times/week. Importantly, this improvement was maintained at the mean 4.6-year follow-up (0.1 ± 0.1 times/week, NS compared to 1-year follow-up). Finally, the incidence of acute respiratory tract infections which averaged 1.4 ± 0.3 events/year prior to device implantation fell to 0.3 ± 0.2 events/year at year 1 and fell further to 0.2 ± 0.1 events/year at the mean 4.6-year mark (NS compared to the 1-year follow-up) (Fig. 5).
Figure 4 .
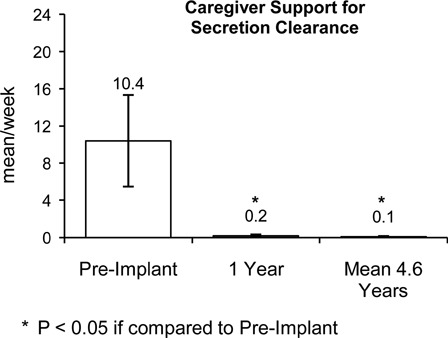
Compared with pre-implant, there were significant reductions in the need for caregiver support to manage secretion clearance at 1-year follow-up (P < 0.05). This reduction was maintained at the mean 4.6-year follow-up (P < 0.05 compared with pre-implant; NS compared to 1-year follow-up).
Figure 5 .

There was a significant reduction in the incidence of acute respiratory tract infections after 1 year of use of the cough stimulation system from 1.4 ± 0.3 to 0.3 ± 0.2 events/year (P < 0.05). The incidence of respiratory tract infection was further reduced at the mean 4.6-year follow-up point to 0.2 ± 0.1 events/year (P < 0.05 compared with pre-implant; NS compared with 1-year follow-up).
Concerning side effects, there were no occurrences of bowel or bladder leakage. There were also no episodes of autonomic dysreflexia. Seven of the 10 subjects continue to experience mild leg jerks with stimulation, but these are painless and do not interfere with use of the device. No other side effects or complications were reported.
Discussion
In a recent clinical trial,1–3 we demonstrated that the SCS system to restore cough is a safe and efficacious method to manage airway secretions. The study protocol involved close monitoring of subjects for a full year following implantation with less intense follow-up thereafter. The results of this study, involving a subset of subjects who had the implant for a minimum of 2 years, clearly demonstrate that the previously observed clinical improvement noted at the 1-year mark was sustained. Moreover, the fact that each subject continued to use the device on a regular basis with perceived clinical benefit and no significant side effects for years following implantation suggests that this device has a high degree of clinical utility. It is also important to note that the cough stimulation system was perceived to be very convenient and reliable and preferable to any other previously employed method of secretion clearance.
Subjective improvements noted by the study participants were reinforced by physiological measurements demonstrating maintenance of the high airway pressure generation achieved with SCS. The observation that airway pressure generation was maintained indicates that these subjects continued to use the device on a regular basis, as lack of use would be expected to result in a decline in expiratory muscle strength to near baseline levels. For example, in studies of phrenic nerve stimulation, discontinuation of pacing for several months results in the redevelopment of disuse atrophy of the diaphragm, as manifested by marked reductions in diaphragm thickness and inspired volume production.4
Potential trial related influences on clinical outcomes
The survey results of the present long-term follow-up study are extremely important to assess for the possibility of potential sources of error inherent in the performance of clinical trials. The initial subjects willing to participate in a clinical trial involving a new device are likely to be highly motivated and optimistic and, therefore, may initially overestimate the clinical benefits of the device in short-term studies.5,6 This is particularly true during the initial phase of the trial during the period of close clinical follow-up in which there is frequent interaction with study staff.
Moreover, studies such as ours which are not blinded and have observational components may also be subject to the Hawthorne effect.7 This effect was originally defined as an increase in worker productivity produced by the psychological stimulus of being singled out and made to feel important.8 The definition was subsequently expanded to medical settings,9 i.e. study results may be affected by the subjects' awareness that they are participating in an investigation and/or receiving special attention. Consequently, this effect can result in an overestimation of treatment effectiveness. The importance of this confounding variable was emphasized by Wolfe and Michaud7 who demonstrated that nearly half of the clinical improvement realized by an FDA approved treatment for rheumatoid arthritis disappeared on entry to a non-sponsored follow-up study, i.e. nearly half the reported improvement in pain and fatigue disappeared. Other investigators evaluating treatments for dementia found that better outcomes were achieved during periods of intensive follow-up compared with those with minimal follow-up.9 In contrast, other studies have found negligible or no such outcomes related to the Hawthorne effect.10
With regard to the SCS method to restore cough, a complete assessment to control for all clinical trial related potential errors would ideally require an assessment of the device in clinical practice. Given the FDA requirements necessary to bring a medical device to market, however, such an analysis is not possible at this time. Nonetheless, re-assessment of the degree of device use and perceptions of benefit following a period of chronic use and under conditions of minimal follow-up, is likely to be more realistic and generalizable to other potential subjects (i.e. real-world setting). Moreover, by the time of re-assessment in this study (mean 4.6 years), potential biases including the initial novelty of the device and potential desire to satisfy the research study staff would have been expected to dissipate completely. We conclude, therefore, that potential trial related effects such as the Hawthorne effect did not have any significant influences on the outcome parameters as measured in our clinical trial.
Study limitations
One obvious weakness of this study is the small number of subjects who met the study criteria and were therefore evaluated. While this limits the strength of our conclusions, this is mitigated somewhat by the fact that each of the study participants implanted with the cough stimulation system, without exception, continued to experience substantial clinical benefits equal to those observed following the initial study period. Moreover, these findings are consistent with long-term users' perceptions of other functional electrical stimulation applications for exercise, standing and transfers after SCI.11 We plan to follow all the remaining subjects already enrolled in the cough stimulation system trial to further substantiate our results.
Another study limitation is the lack of control group. Ideally, a rigorous study design would include a matched control group. However, this is an extremely heterogeneous group of subjects (i.e. age, sex, weight, duration of injury, injury level, incidence of infections, co-morbidities, and others) in an orphan population. Consequently, a matched control group is not practically feasible. Therefore, in our pilot study, subjects served as their own control. This is typical for studies involving orphan populations and in the evaluation of functional electrical stimulation (FES) devices.12,13
Further, randomization was not part of the study design. Blinding cannot be practically implemented since the muscle activity produced by electrical stimulation, in what are otherwise completely paralyzed individuals, cannot be masked from either the subject or evaluator. Given the highly significant results of this study, the lack of a control group does not undermine the importance of our findings.
Future directions
While use of the SCS device to restore cough appears to impart a high degree of clinical benefit, an invasive procedure involving a several hour surgical procedure is required for placement of the disc electrodes. This factor is likely to impede widespread application of the device, as several prospective subjects in our recent clinical trial declined participation for this reason. Moreover, this requirement is likely to impede physician acceptance and referral for this procedure, as well.
Recent studies performed in animals, however, suggest that electrode placement can also be accomplished using wire lead technology.14 The significant advantage of these types of leads is that they can be inserted percutaneously via minimally invasive techniques through a needle. It should be noted that the placement of spinal cord stimulators using wire leads for control of pain is already commonplace and has been in clinical use for more than 2 decades.15–21 Given the broad experience and safety record of these types of percutaneous leads, therefore, use of these systems to restore an effective cough could theoretically be readily adapted into clinical practice. Obviously a clinical trial will be necessary to assess the safety and efficacy of wire lead technology to restore cough in subjects with SCI.
Conclusions
SCS to restore cough results in significant clinical benefits related to secretion management in subjects with SCI. The clinical benefits related to ease in raising secretions, reduction in the need for caregiver support and reduction in the incidence of respiratory infections observed after 1 year were maintained after a mean 4.6 years of use. Moreover, airway pressure generation during SCS was maintained over the entire study period corroborating subjects' responses of continued regular use of the device. Taken together, these results argue in favor of substantial long-term benefit of this clinical modality. The fact that 100% of subjects continued to use the device during a period of minimal investigational oversight indicates that the initial clinical benefits were not secondary to potential clinical trial related errors and may be generalizable to real-world clinical settings.
Acknowledgment
The authors gratefully acknowledge the technical assistance in data analysis of statistician, Charles Thomas, B.A.
References
- 1.DiMarco AF, Kowalski KE, Geertman RT, Hromyak DR. Spinal cord stimulation: a new method to produce cough in patients with spinal cord injury. Am J Respir Crit Care Med 2006;173:1386–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DiMarco AF, Kowalski KE, Geertman RT, Hromyak DR. Lower thoracic spinal cord stimulation to restore cough in patients with spinal cord injury: results of a National Institutes of Health-sponsored clinical trial. Part I: methodology and effectiveness of expiratory muscle activation. Arch Phys Med Rehabil 2009;90:717–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DiMarco AF, Kowalski KE, Geertman RT, Hromyak DR, Frost FS, Creasey GH, et al. Lower thoracic spinal cord stimulation to restore cough in patients with spinal cord injury: results of a National Institutes of Health-sponsored clinical trial. Part II: clinical outcomes. Arch Phys Med Rehabil 2009;90:726–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayas NT, McCool FD, Gore R, Lieberman SL, Brown R. Prevention of human diaphragm atrophy with short periods of electrical stimulation. Am J Resp and Crit Care Med 1999;159:2018–20 [DOI] [PubMed] [Google Scholar]
- 5.Hawthorne G, Hogan A, Giles E, Stewart M, Kethel L, White K, et al. Evaluating the health-related quality of life effects of cochlear implants: a prospective study of an adult cochlear implant program. Int J Audiol 2004;43:183–92 [DOI] [PubMed] [Google Scholar]
- 6.Carroll DL, Hamilton GA. Long-term effects of implanted cardioverter-defibrillators on health status, quality of life, and psychological state. Am J Crit Care 2008;17:222–30 [PubMed] [Google Scholar]
- 7.Wolfe F, Michaud K. The Hawthorne Effect, sponsored trials, and the overestimation of treatment effectiveness. J Rheumatol 2010;37:2216–20 [DOI] [PubMed] [Google Scholar]
- 8.Franke RH, Kaul JD. The Hawthorne experiments: first statistical interpretation. Am Soc Rev 1978;43:623–43 [Google Scholar]
- 9.McCarney R, Warner J, Iliffe S, van Haselen R, Griffin M, Fisher P. The Hawthorne Effect: a randomized, controlled trial. BMC Med Res Methodol 2007;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernald DH, Coombs L, DeAlleaume L, West D, Parnes B. An assessment of the Hawthorne Effect in practice-based research. J Am Board Fam Med 2012;25:83–6 [DOI] [PubMed] [Google Scholar]
- 11.Agarwal S, Triolo RJ, Kobetic R, Miller M, Bieri C, Kukke S, et al. Long-term user perceptions of an implanted neuroprosthesis for exercise, standing, and transfers after spinal cord injury. J Rehabil Res Dev 2003;40:241–52 [PubMed] [Google Scholar]
- 12.DiMarco AF Phrenic nerve stimulation in patients with spinal cord injury. Respir Physiol Neurobiol 2009;169:200–9 [DOI] [PubMed] [Google Scholar]
- 13.Nemunaitis G, Kilgore K, Triolo R, Kobetic R, Creasey G, DiMarco A. Neuromuscular electrical stimulation in spinal cord injury. In: Kirschblum S, Campagnolo D, Delisa J, et al. (eds.) Spinal cord injury medicine. 2nd ed Philadelphia: Lippincott, Williams & Wilkins; 2011. Ch. 25 [Google Scholar]
- 14.Kowalski KE, DiMarco AF. Comparison of wire and disc leads to activate the expiratory muscles in dogs. J Spinal Cord Med 2011;34:600–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett DS, Alo KM, Oakley J, Feler CA. Spinal cord stimulation for complex regional pain syndrome I [RSD]: a retrospective multicenter experience from 1995 to 1998 of 101 patients. Neuromodulation 1999;2:202–10 [DOI] [PubMed] [Google Scholar]
- 16.Burchiel KJ, Anderson VC, Brown FD, Fessler RG, Friedman WA, Pelofsky S, et al. Prospective, multicenter study of spinal cord stimulation for relief of chronic back and extremity pain. Spine 1976;21:2786–94 [DOI] [PubMed] [Google Scholar]
- 17.Cameron T Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J Neurosurg 2004;100:254–67 [DOI] [PubMed] [Google Scholar]
- 18.Kumar K, Hunter G, Demeria D. Spinal cord stimulation in treatment of chronic benign pain: challenges in treatment planning and present status, a 22-year experience. Neurosurgery 2006;58:481–96 [DOI] [PubMed] [Google Scholar]
- 19.North RB, Kidd DH, Petrucci L, Dorsi MJ. Spinal cord stimulation electrode design: a prospective, randomized, controlled trial comparing percutaneous with laminectomy electrodes: Part II clinical outcomes. Neurosurgery 2005;57:990–6 [DOI] [PubMed] [Google Scholar]
- 20.Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg 1967;46:489–91 [PubMed] [Google Scholar]
- 21.Taylor RS, Taylor RJ, Van Buyten JP, Buchser E, North R, Bayliss S. The cost effectiveness of spinal cord stimulation in the treatment of pain: a systematic review of the literature. J Pain Symptom Manage 2004;27:370–8 [DOI] [PubMed] [Google Scholar]


