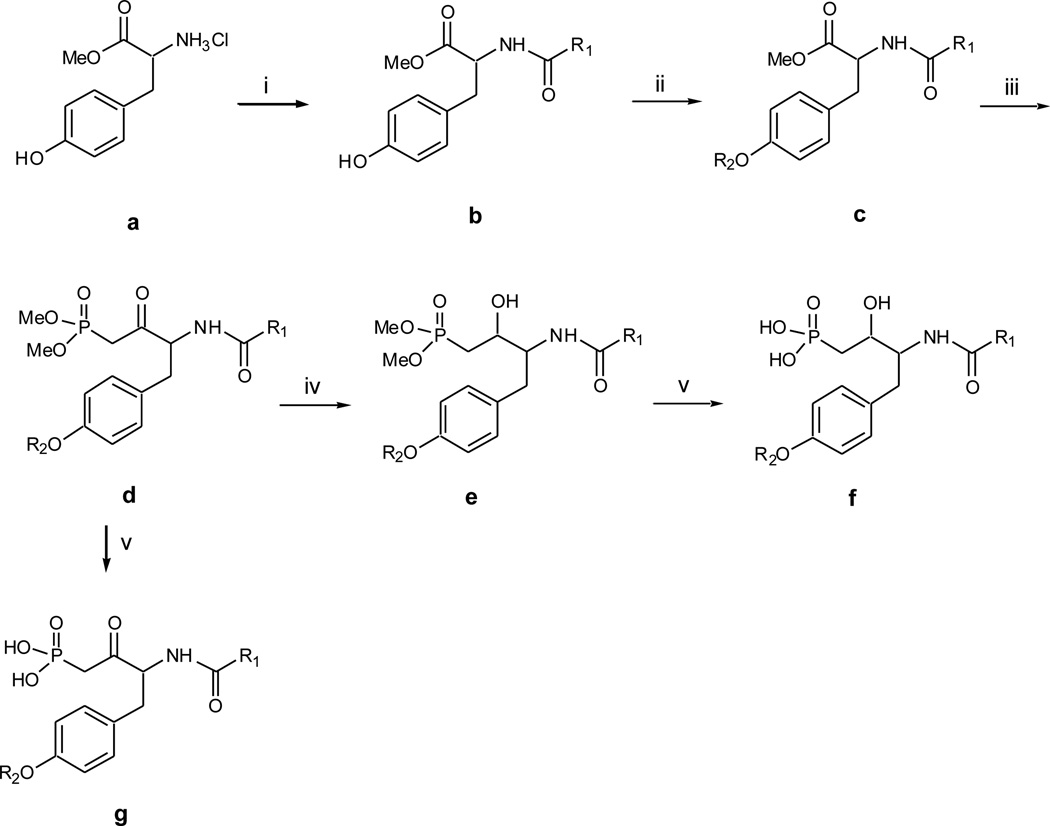
Scheme 1.
Synthesis of Compounds f and g. Reagents and conditions: (i) appropriate acyl chloride, Et3N, CH2Cl2, 0°C, 3hr, 70–80%; (ii) appropriate mesylate, K2CO3, 18-crown-6, acetone, reflux overnight, 90–95%; (iii) n-BuLi, dimethyl methylphosphonate, then add in ester c, −78°C, 3hr, 50–60%; (iv) NaBH4, THF, EtOH, 0°C, 2hr, 70–80%; (v) bromotrimethylsaline, w/wo pyridine, CH2Cl2, rt, 4hr, then H2O and MeOH, overnight, 90–95%.