Table 3.
ATX inhibitory evaluation of compound f35 – f44
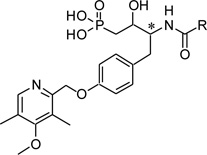 | |||||
|---|---|---|---|---|---|
| Compounds | R | * S/R | ATX activity (%) | ||
| 1 µM | 10 µM | 10 µM | |||
| f35 | n-C9H19 | R (a1) | N/D | 86 | 30 |
| f36 | n-C9H19 | R (b1) | N/D | N/D | N/D |
| f37 | n-C13H27 | R (a) | N/D | 99 | 67 |
| f38 | n-C13H27 | R (b) | N/D | N/D | 35 |
| f39 | n-C17H35 | S (a) | 101 | 91 | 81 |
| f40 | n-C17H35 | S (b) | N/D | 90 | 62 |
| f41 | n-C17H332 | S (a) | N/D | 45 | 15 |
| f42 | n-C17H332 | S (b) | 77 | 68 | 58 |
| f43 | n-C19H39 | S (a) | 64 | 45 | 10 |
| f44 | n-C19H39 | S (b) | N/D | N/D | N/D |
a refers to the diastereomer that elutes first, b refers to the diastereomer that elutes second.
cis double bond located between C9 and C10 from the carbonyl.
