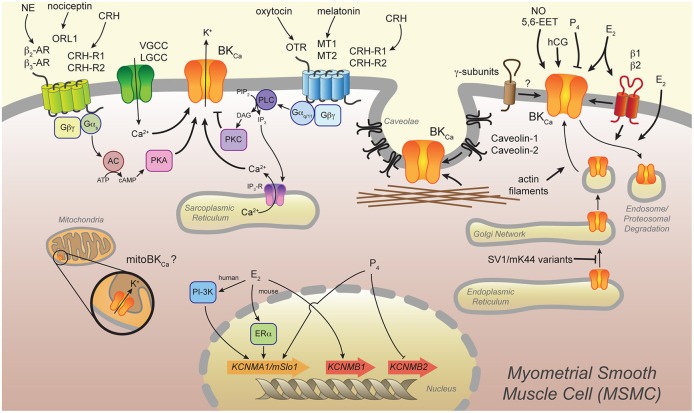
Figure 1.
Several mechanisms modulate the BKCa channel in the myometrium. Certain splice variants (SV1 and mK44) of the BKCa channel are retained in the endoplasmic reticulum, whereas actin filaments induce traffic of BKCa to the plasma membrane of the myometrial smooth muscle cell (MSMC). Localization of BKCa channels in membrane microdomains (i.e., caveolae) and interaction with caveolin-1 and -2 and actin filaments modulate the channel's activity. The BKCa auxiliary β1- and β2-subunits modify channel activation by direct interaction and, in the case of β1, by inducing its internalization to endosomes. Novel BKCa auxiliary γ-subunits are expressed in the uterus, but their significance for MSMC excitability has not been assessed. The vasoactive molecules nitric oxide (NO) and epoxyeicosatrienoic acid (5,6-EET) induce relaxation of the myometrium likely by modulation of BKCa channel activity. The steroid hormones 17β-estradiol (E2) and progesterone (P4) are important in maintaining pregnancy and inducing labor. These hormones modulate activity of the BKCa channel in several ways: directly modulating BKCa channel activity, inducing proteosomal degradation of the channel, and regulating expression of the genes encoding the BKCa α-subunit (KCNMA1/mSlo1) or β-subunits (KCNMB1 and KCNMB2). Another pregnancy-related hormone, human chorionic gonadotropin (hCG), modulates BKCa channel activity to induce relaxation of the myometrium. Several G-protein coupled receptors (GPCRs) regulate BKCa channel activity in MSMCs. Norepinephrine (NE) and nociceptin bind their receptors, β2- and β3-adrenoceptors (β2- and β3-AR) and the orphan opioid receptor-like 1 (ORL-1), respectively, and thereby activate G-proteins (Gαs, Gβγ). This leads to adenylyl cyclase (AC) production of cyclic AMP (cAMP), which activates protein kinase A (PKA) and modulates BKCa channel activity. Oxytocin and melatonin stimulate oxytocin receptor (OTR) and melatonin receptors 1 and 2 (MT1 and MT2), respectively, and thereby induce Gαq/11-dependent activation of phospholipase C (PLC). This leads to production of diacylglycerol (DAG), which in turn causes protein kinase C (PKC)-dependent phosphorylation of the BKCa channel. PLC also produces inositol 1,4,5-triphosphate (IP3) from membrane-bound phosphatidylinositol 4,5-bisphosphate (PIP2) and thereby brings about Ca2+ release from the sarcoplasmic reticulum. In addition to activation by Ca2+ release from intracellular stores, the BKCa channel is activated by Ca2+ influx from nearby voltage- or ligand-gated Ca2+ channels (VGCC and LGCC, respectively). Corticotropin-releasing hormone (CRH) binds to its receptors CRH-R1 and CRH-R2, which are linked to multiple signaling pathways and induce up- or down-regulation of BKCa channel activity. Finally, a particular BKCa channel (mitoBKCa) targets to the inner membrane of mitochondria and may influence MSMC contractility.