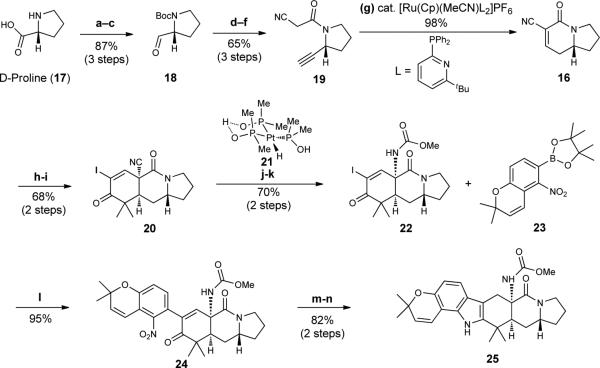
Figure 3. Preparation of fused hexacycle 25.
The use of a Diels-Alder reaction involving a proline-derived indolizidinone dienophile affords a key tricycle that is advanced to hexacycle 25 by Suzuki coupling to boronic ester 23. Reagents and conditions are as follows. a. di-tert-butyl dicarbonate (Boc2O), NaHCO3, H2O/tetrahydrofuran (THF), room temperature (RT = 23 °C). b. BH3•THF, THF, 0 °C to RT. c. (COCl)2, dimethylsulfoxide (DMSO), CH2Cl2, diisopropylethylamine (DIPEA), − 78 °C. d. Dimethyl (diazomethyl)phosphonate, K2CO3, MeOH, 0 °C to RT. e. 4N HCl/Dioxane, 0 °C to RT. f. 2-cyanoacetylchloride, Et3N, CH2Cl2, 0 °C to RT. g. acetonitrile bis[2-diphenylphosphino-6-t-butylpyridine] cyclopentadienylruthenium(II) hexafluorophosphate (8 mol%), acetone/H2O, 70 °C. h. 15, SnCl4, −78 °C to −42 °C. i. I2, 4-dimethylaminopyridine (DMAP), pyridine/CCl4, 60 °C. j. 21 (20 mol%), EtOH/H2O, RT. k. phenyliodosylbistrifluoroacetate (PIFA), MeOH, RT. l. dppfPdCl2 (10 mol%), K3PO4, dimethylformamide (DMF), 40 °C. m. Zn dust, NH4Cl, HCO2NH4, p-TsOH, MeOH, RT; n. NaCNBH3, 1 N aq. HCl, 0 °C to RT. dppf, diphenylphosphinoferrocene; Me, methyl.