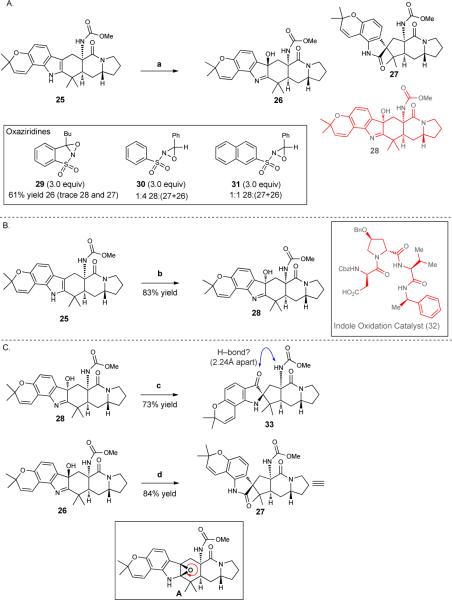
Figure 4.
A. Oxidative rearrangement studies of fused indole 25 with a range of oxaziridines leads predominantly to the undesired, epimeric, hydroxyindolenine (26) and spirooxindole (27). Figure 4B. Use of indole oxidation peptide catalyst 32 to effect oxidation yields the desired hydroxyindolenine (28). Figure 4C. The desired hydroxyindolenine 28 rearranges to an undesired pseudoindoxyl (33) whereas the epimeric hydroxyindolenine (26) affords the corresponding spirooxindole (27). Reagents and conditions are as follows. a. oxaziridine (29, 30, or 31), CH2Cl2, room temperature (RT = 23 °C). b. 32 (20 mol%), 4-dimethylaminopyridine (DMAP), diisopropylcarbodiimide (DIC), H2O2, CHCl3, 4 °C. c. Sc(OTf)3, toluene, 110 °C. d. 23 mM HCl, CH2Cl2, RT. Me, methyl; Bu, butyl; Bn, benzyl; Cbz, carboxybenzyl; Ph, phenyl.