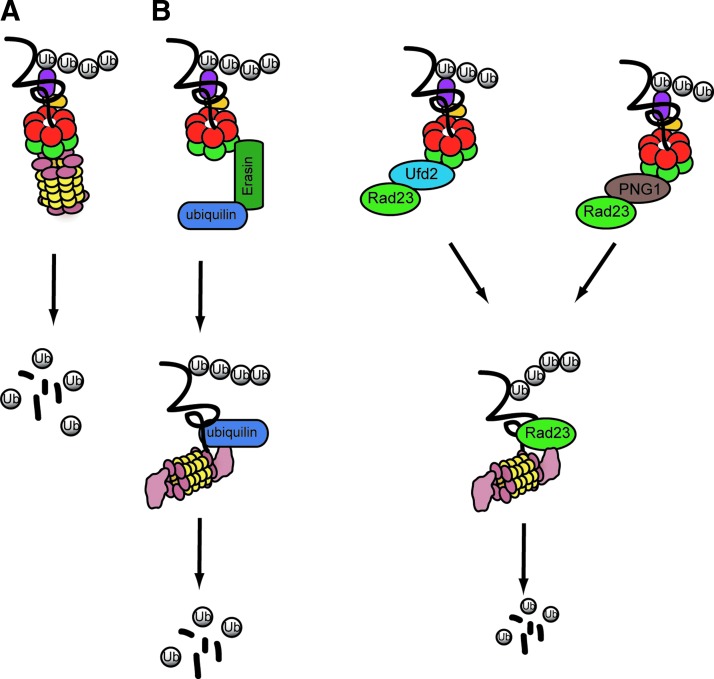
FIG. 2.
Distinct mechanisms for transferring proteins from p97/VCP to the proteasome. (A) The direct docking model. Studies on archaeal Cdc48 showed direct interaction between Cdc48 and the 20S proteasome, suggesting a possible direct path from p97/Cdc48 to the proteolytic proteasome chamber for substrates. (B) Substrates are transferred from p97/Cdc48 to the proteasome using different cofactors that interact with p97/Cdc48 and the proteasome. (1) p97/Cdc48 interacts with erasin, which recruits ubiquilin. Then, the substrate is transferred to the proteasome by ubiquilin. (2) p97/Cdc48p binds to E4 ligase (E4B in mammalian cells and Ufd2p in yeast), which recruits Rad23p to p97/Cdc48p. Rad23p then passes the substrates to the proteasome. For glycosylated substrates, PNGase 1 (PNG1) may facilitate the transfer of substrates from p97/Cdc48 to Rad23.