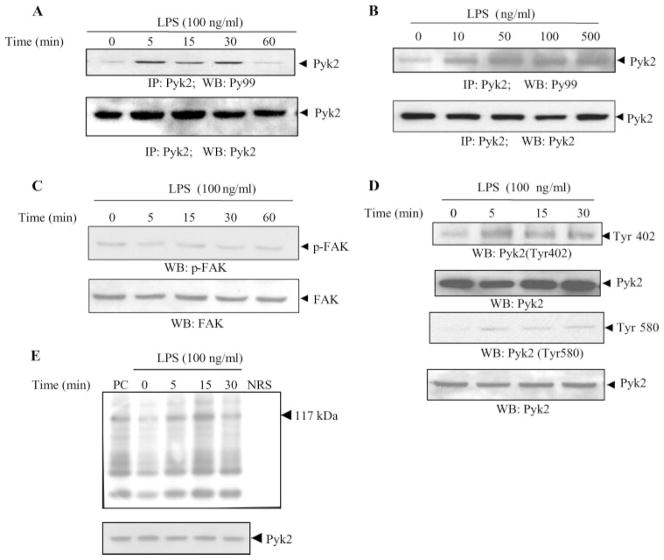
FIGURE 1.
LPS induces tyrosine phosphorylation and enzymatic activity of Pyk2 in endothelial cells. HUVEC were stimulated with LPS (100 ng/ml) for the indicated periods of time (A) or with various concentrations of LPS for 15 min (B). The lysates were then immunoprecipitated with Abs to Pyk2. The immunoprecipitates were analyzed by Western blotting with Abs to phosphotyrosine (Py99). The same blot was then probed with anti-Pyk2 Ab. C and D, Lysates obtained from HUVEC stimulated with LPS (100 ng/ml) for various periods of time were also analyzed by Western blotting with Abs to phospho-FAK (p-FAK) (C) or with Abs to phospho-Pyk2 (Tyr 402) and phospho-Pyk2 (Tyr 580) (D). E, The kinase activity in the immunoprecipitates was assessed as the ability of Pyk2 to phosphorylate the synthetic substrate poly(Glu-Tyr)4:1. A typical autoradiograph is shown (117 kDa). (top). NRS, Normal rabbit serum; PC, positive control. The total abundance of Pyk2 protein was analyzed by Western blot analysis of the lysates, as indicated (bottom). Data show one representative experiment of three independent experiments performed.