Abstract
Introduction
Capasio is being developed as a new generation of endodontic material with potential use as a root-end filling material. The aim of this study was to compare the ability of Capasio and mineral trioxide aggregate (MTA) to penetrate human dentinal tubules and examine the interaction of Capasio and MTA with a synthetic tissue fluid (STF) and root canal walls in extracted human teeth.
Methods
Root-end preparations were filled with Capasio or MTA, allowed to set for 4 weeks in STF, and then sectioned at 1, 2, and 3 mm from resected surface. Depth of penetration was evaluated by using scanning electron microscopy (SEM). Next, Capasio and MTA samples were prepared both in 1-g pellets and in root-end preparations. Samples were placed in STF, allowed to set, and then characterized by using SEM, energy dispersive x-ray analysis (EDXA), and x-ray diffraction (XRD) techniques.
Results
Penetration of Capasio into dentinal tubules was observed at all levels. No penetration of MTA into dentinal tubules was observed at any level. Both Capasio and MTA formed apatite crystals in the supernatant, on their exposed surfaces, and in the interfacial layers that were similar in structure and elemental composition when evaluated by using SEM and EDXA. XRD analysis of these crystals corresponds with those reported for hydroxyapatite.
Conclusions
When used as a root-end filling material, Capasio is more likely to penetrate dentinal tubules. Both Capasio and MTA promote apatite deposition when exposed to STF.
Keywords: Biomineralization, Capasio, carbonated apatite, MTA, synthetic tissue fluid, tubule penetration
Endodontic retreatment of teeth that have not responded to initial nonsurgical root canal treatment can result in successful outcomes ranging from 56%–84% (1, 2). However, recent advances in surgical techniques, equipment, and materials have made endodontic surgery a predictable treatment option in cases that have not responded to initial endodontic therapy or when nonsurgical root canal therapy is contraindicated (3-5). A recent retrospective study has shown that several factors such as sex, tooth position, lesion type, and root-end filling material are likely predictors of positive clinical outcomes (6). Thus, the long-term success of endodontic surgery is often influenced by the type of root-end filling material used (3, 6, 7).
The primary objective of endodontic treatment is to eliminate microorganisms and prevent reinfection (8). However, it is impossible to completely eliminate all microorganisms from the canal space (9). Therefore, an ideal root-end filling material should closely adapt to the margins of the canal, display dimensional stability, demonstrate adequate bond strength, eliminate avenues of leakage, entomb remaining bacteria, and exhibit biocompatibility with host tissues (8, 10-14). Studies have demonstrated that penetration of an endodontic material into the dentinal tubules will improve marginal adaptation (15), increase mechanical retention (8, 15), entomb residual bacteria (8), and exert antibacterial effects because of closer proximity of material to the bacteria (16).
A variety of retrofilling materials are currently used for periradicular surgery. Specifically, white ProRoot mineral trioxide aggregate (MTA) (Dentsply Tulsa Dental Specialties, Tulsa, OK) has gained popularity among endodontists since its introduction in 1993 (3, 17). The sealing ability (18), osteogenic potential (19), and biocompatibility (20-22) of MTA have been attributed to its ability to release calcium ions, form an adherent interfacial layer, and trigger the precipitation of hydroxyapatite on its surface and in the surrounding fluid (23). However, MTA can be difficult to manipulate (24), perceived as coarse (25), slow to set (26), and easily washed out (27, 28).
A nonrandomized prospective clinical study that used endodontic lesions limited to the periapical region found that MTA-treated teeth demonstrated a significant improvement in healing in comparison with Retroplast-treated teeth (29). In addition, the MTA-treated group had a higher percentage of cases considered to be healed compared with the Retroplast-treated teeth, particularly in mandibular premolars and molars.
Although current root-end filling materials have shown varying degrees of successful outcomes, no single material exhibits all characteristics of an ideal root-end filling material. New root-end filling materials are consistently being introduced in an effort to improve on the weaknesses of previous materials. Capasio (Primus Consulting, Bradenton, FL) is a calcium-phospho-aluminosilicate–based cement that uses a novel setting reaction and has demonstrated similar or improved physical characteristics such as setting time, radiopacity, compressive strength, pH, and washout resistance (27). Such favorable properties make Capasio a potential root-end filling material; however, evidence is still lacking to justify the use of this material as an improvement over MTA.
The purposes of this study were to evaluate the ability of Capasio and MTA to penetrate human dentinal tubules when used as a root-end filling material and to examine the interaction of Capasio and MTA with synthetic tissue fluid (STF) and endodontically prepared root canal walls in extracted human teeth. STF was chosen to simulate the in vivo conditions in which rootend filling materials are used.
Materials and Methods
Penetration of Material into Dentinal Tubules
Thirty intact, single-rooted, anterior human mature teeth were selected for the study and stored in 10% buffered formalin. Teeth were matched in pairs on the basis of tooth type and anatomy and then randomly assigned into 2 groups of 15 teeth. The teeth were decoronated at the cementoenamel junction. A size 10 FlexoFile (Dentsply/Maillefer, Tulsa, OK) was inserted into each canal until it exited the apical foramen, and the length was measured. Working length was established by subtracting 0.5 mm from this length. The canals were endodontically prepared by a single operator who used rotary nickel-titanium ProFile instruments (Dentsply/Maillefer) to a master apical file size of 50/04. A 3.0-mm root-tip resection was performed 90° to its long axis with a high-speed, Multi-Purpose bur (Dentsply/Maillefer) under water cooling. A 4.0-mm-deep apical preparation was cut by using ultrasonic tips (KiS Microsurgical Instruments; Obtura/Spartan, Fenton, MO) powered by an ultrasonic unit (Satelec P5; Dentsply Tulsa, Tulsa, OK). All canals were irrigated by using a PiezoFlow ultrasonic irrigation needle (Dentsply Tulsa) alternating between a total of 10 mL 6% NaOCl and 5 mL 17% ethyl-enediaminetetraacetic acid (EDTA). Thermoplasticized gutta-percha (Obtura/Spartan) without sealer was placed in the canal system except in the apical 5 mm, which allowed material to be adequately compacted.
Experimental Capasio and MTA were compared. Group 1 was filled with Capasio mixed according to the manufacturer’s recommendation at a weight ratio of 4:1 powder to gel. Group 2 was filled with MTA mixed according to the manufacturer’s recommendation at a weight ratio of 3:1 powder to sterile water. Materials were compacted into root-end preparations in small increments by using microsurgical pluggers (KiS Microsurgical Instruments; Obtura/Spartan). Filled teeth were placed in individual vials containing 3 mL of STF. The STF was a phosphate-buffered saline solution (pH 7.2) with a composition of 1.7 g KH2PO4, 11.8 g Na2HPO4, 80.0 g NaCl, and 2.0 g KCl in 10 L of H2O. After 4 weeks at 37°C and 100% humidity, each tooth was sliced at 90° to the long axis of the tooth by using a diamond blade, removing three 1-mm sections beginning 1 mm from the resected surface. Each surface was demineralized with a 10-minute application of 17% EDTA, followed by a 10-minute application of 6% NaOCl. Sections were washed with distilled water, mounted, and dried. Sections were then prepared for scanning electron microscopy (SEM) evaluation by sputter-coating the surface with a thin gold coating. Examination for material penetration into dentinal tubules was completed for each section. The depth of material penetration was measured at 10 locations around the canal’s circumference by using a calibrated measuring tool incorporated into the microscope control system. The mean, minimum, and maximum penetration for each section was calculated. Statistical analysis was performed by using the χ2 test.
Interaction of Material with STF
Ten samples of MTA material and 10 samples of Capasio material were mixed according to the manufacturers’ recommendations. Each 1.0-g sample was mixed and placed in a custom mold (diameter of 15.0 mm), with the top surface exposed. A total of 25 mL of STF was immediately added to each sample. All samples were stored at 37°C and 100% humidity for 4 weeks. The supernatant of each sample was poured off, filtered, washed, and dried. Set samples were dried. Precipitates attached to the exposed surface were removed and collected. All samples were prepared for evaluation by SEM, energy dispersive x-ray analysis (EDXA), and x-ray diffraction (XRD) techniques.
Interaction of Material with STF and Prepared Root Canal Walls of Extracted Teeth
Twelve single-rooted, human extracted teeth were prepared as described earlier. Teeth were matched in pairs on the basis of tooth type and anatomy. Teeth were randomly assigned into 2 groups of 5 teeth each. Five teeth were filled with MTA and 5 teeth were filled with Capasio by using the mixing ratios and techniques previously described. Two teeth served as the control group and were completely filled with gutta-percha. Teeth were immediately placed into 10 mL STF and stored for 2 months at 37°C and 100% humidity. All samples were then removed, dried, and prepared for evaluation by SEM and EDXA.
Results
Penetration of Material into Dentinal Tubules
Group 1 containing Capasio as a root-end filling had dentinal tubule penetration in 17 of 35 samples (Fig. 1). No penetration into the dentinal tubules was observed at any level in group 2 with ProRoot MTA as the root-end filling. Chi-squared tests showed an overall significant difference between the Capasio and MTA groups (P < .001).
Figure 1.
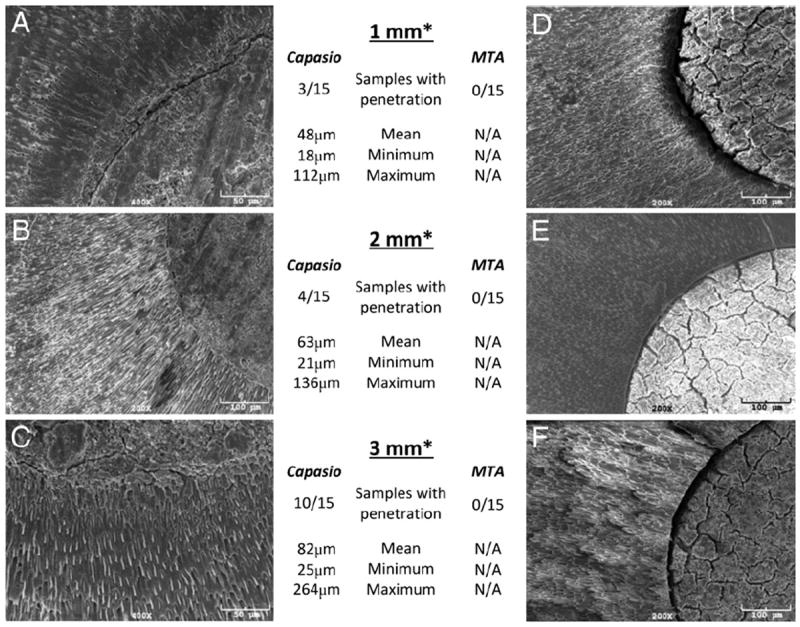
(A–C) SEM images of Capasio-dentin interface from group 1. At (A) 1 mm, (B) 2 mm, and (C) 3 mm from resected root-end, extensive Capasio tags are seen penetrating into the dentinal tubules. (D–F) SEM images of ProRoot MTA-dentin interface from group 2. At (D) 1 mm, (E) 2 mm, and (F) 3 mm from resected root-end, no MTA tags are seen penetrating the dentinal tubules. Number of samples with material penetration presented with corresponding mean, minimum, and maximum. *From apical resected surface.
Penetration increased with distance from the resected surface. In the Capasio group at 1 mm from the resected surface, 20% of samples showed material within dentinal tubules, with a mean penetration depth of 48 μm. At 2 mm from the resected surface, 26% of samples showed material in dentinal tubules, with a mean penetration depth of 63 μm. At 3 mm from the resected surface, 66% of samples showed material in dentinal tubules, with a mean penetration of 82 μm (Fig. 1).
Interaction of Material with STF
In group 1, SEM evaluation of both the precipitate from the supernatant and the precipitate from the exposed surface of the material revealed individual globules localized in clusters. EDXA indicated that these precipitates contained primarily Ca (18%), P (15%), Si (1%), Al (17%), and O (52%). Similarly, group 2 revealed individual globules localized in clusters in both precipitates. EDXA results for the MTA precipitates closely aligned with those for the Capasio precipitates, because MTA contained primarily Ca (26%), P (16%), Si (6%), and O (38%). XRD analysis of both Capasio and MTA precipitates had diffraction lines for hydroxyapatite (HA) (Fig. 2B).
Figure 2.
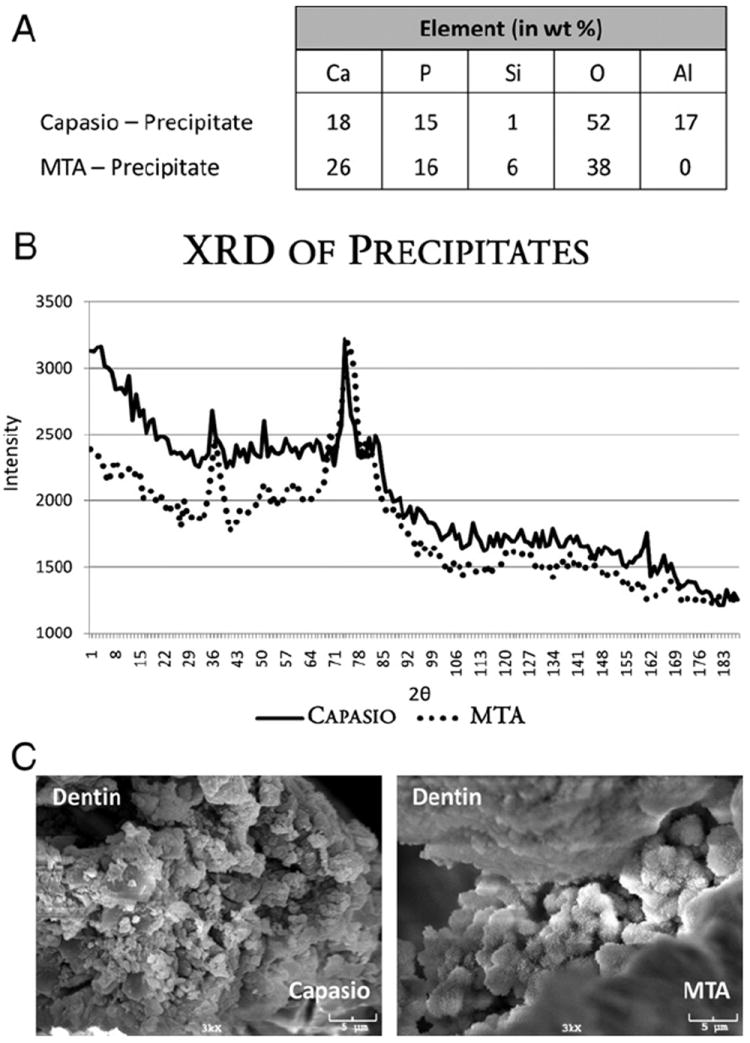
(A) EDXA spectrum from Capasio and MTA precipitates. (B) XRD pattern of Capasio and MTA STF precipitates. Intensity peaks match International Centre for Diffraction Data PDF for HA (34-0010 and 24-0033). (C) SEM images of dentin/material interfaces for Capasio and MTA.
Interaction of Material with STF and Prepared Root Canal Walls of Extracted Teeth
SEM examination was conducted on both the MTA-dentin and the Capasio-dentin interfaces. Each sample had a definitive separation between the material and the dentin, most likely because of desiccation and high vacuum associated with SEM. However, each sample contained areas demonstrating an interfacial layer without gaps at the interface. Under 3000× magnification, the interfacial layer appeared as globules creating a latticework to connect the material to the dentin structure (Fig. 2C). EDXA of these clusters showed the presence of Ca, P, Si, and O. These globules were similar in their elemental composition to the precipitates outlined in Figure 2A. In the control group, there was a separation between gutta-percha and dentin without an interfacial layer.
Discussion
Previous studies have used SEM to evaluate the depth of penetration of endodontic materials into dentinal tubules (8, 15, 30, 31). SEM allows for detailed observation of material penetration at distant sites from the canal wall and accurate measurement of penetration. An SEM study by Dreger et al (32) examined the intratubular mineral deposition into dentinal tubules after interaction with MTA or Portland cement. MTA was determined to be more effective in promoting biomineralization at the dentin-cement interface.
In our study no penetration of MTA into dentinal tubules was observed; however, Capasio demonstrated penetration depths ranging from 18–264 μm. The ability of an endodontic material to penetrate dentinal tubules can be attributed to the number of dentinal tubules, the size of dentinal tubules, the particle size of the material, and the setting reaction of the material. Dentinal tubules are structures that range in diameter from 2.0–3.2 μm at the pulpal wall (33). The number of tubules is greatest in cervical dentin, with a significant reduction in the average density of tubules in radicular dentin (34). For material to penetrate the tubules, the particle size must be smaller than the diameter of the tubule. The mean particle size for white ProRoot MTA is 10 μm, with all particles being smaller than 50 μm (35). In contrast, Capasio has a mean particle size of 5.3 μm, with all particles being smaller than 20 μm and about 5% finer than 1 μm (personal communication, Dr Carolyn Primus, August 17, 2011). The finer powder would allow transport into the tubules. An alternative explanation is that dissolution and reprecipitation of the Capasio powder allowed the filling of the dentinal tubules.
Both Capasio and MTA demonstrated an ability to precipitate apatite crystals on their surface, in interfacial layers, and in the surrounding fluid. Previous MTA studies have assessed the apatite layer formed on its surface, in the interfacial spaces, and in the dentinal tubules. These studies concluded that this physiochemical reaction results in the gradual formation of HA between theMTA and dentin that improves sealing ability and biocompatibility (15, 23). Cell viability has been shown to be linked to factors such as the type of endodontic material used, setting time, and incubation time (36). Therefore, the assessment of any new material and its ability to maintain biocompatibility must account for a multitude of factors when determining whether it will be effective.
The formation of apatite is driven primarily by the release of calcium into biologic fluids. MTA has been shown to promote the release of calcium into the hard tissues that form beneath it when used as a capping material (17). Other materials that release calcium have been shown to elicit biologic responses similar to that of MTA, such as calcium hydroxide, calcium phosphate cement, HA cement, and Portland cement (37-40). Capasio formed apatite crystals in the supernatant, on its exposed surface, and in the interfacial layers. Aluminum detected in Capasio samples might be attributed to the material being incorporated into the crystals or from material adjacent to crystals. Overall, XRD analysis of these crystals corresponds with those reported for HA.
When used as a root-end filling material, Capasio is more likely to penetrate dentinal tubules and equally likely to promote apatite deposition when compared with MTA. However, further studies are required to recommend Capasio as a material for clinical use.
Acknowledgments
The authors thank the AAE Foundation for their support of this research, Dr Primus for her input, and Ms Connie Tillberg for assistance in specimen preparation. The authors gratefully acknowledge Tulsa Dental and Primus Consulting for supplying the materials.
Dr Opperman is a consultant on NIDCR grant 1R43DE020204-01A1 for Primus Consulting, Bradenton, Florida, which has a direct financial interest in the subject or materials discussed in this article. Capasio used in this study is being developed through 1R43DE020204-01A1 funding.
References
- 1.Bergenholtz G, Lekholm U, Milthon R, Heden G, Odesjo B, Engstrom B. Retreatment of endodontic fillings. Scand J Dent Res. 1979;87:217–24. doi: 10.1111/j.1600-0722.1979.tb00675.x. [DOI] [PubMed] [Google Scholar]
- 2.Gorni FG, Gagliani MM. The outcome of endodontic retreatment: a 2-yr follow-up. J Endod. 2004;30:1–4. doi: 10.1097/00004770-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Kim S, Kratchman S. Modern endodontic surgery concepts and practice: a review. J Endod. 2006;32:601–23. doi: 10.1016/j.joen.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Rubinstein RA, Kim S. Long-term follow-up of cases considered healed one year after apical microsurgery. J Endod. 2002;28:378–83. doi: 10.1097/00004770-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Zuolo ML, Ferreira MO, Gutmann JL. Prognosis in periradicular surgery: a clinical prospective study. Int Endod J. 2000;33:91–8. doi: 10.1046/j.1365-2591.2000.00263.x. [DOI] [PubMed] [Google Scholar]
- 6.Song M, Jung I-Y, Lee S-J, Lee C-Y, Kim E. Prognostic factors for clinical outcomes in endodontic surgery: a retrospective study. J Endod. 2011;37:927–33. doi: 10.1016/j.joen.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Gutmann JL, Harrison JW. Surgical endodontics. Boston, MA: Blackwell Scientific Publications; 1991. [Google Scholar]
- 8.Mamootil K, Messer HH. Penetration of dentinal tubules by endodontic sealer cements in extracted teeth and in vivo. Int Endod J. 2007;40:873–81. doi: 10.1111/j.1365-2591.2007.01307.x. [DOI] [PubMed] [Google Scholar]
- 9.Peters LB, Wesselink PR. Periapical healing of endodontically treated teeth in one and two visits obturated in the presence or absence of detectable microorganisms. Int Endod J. 2002;35:660–7. doi: 10.1046/j.1365-2591.2002.00541.x. [DOI] [PubMed] [Google Scholar]
- 10.Gartner AH, Dorn SO. Advances in endodontic surgery. Dent Clin North Am. 1992;36:357–78. [PubMed] [Google Scholar]
- 11.Kratchman SI. Perforation repair and one-step apexification procedures. Dent Clin North Am. 2004;48:291–307. doi: 10.1016/j.cden.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Lee YL, Lee BS, Lin FH, Yun Lin A, Lan WH, Lin CP. Effects of physiological environments on the hydration behavior of mineral trioxide aggregate. Biomaterials. 2004;25:787–93. doi: 10.1016/s0142-9612(03)00591-x. [DOI] [PubMed] [Google Scholar]
- 13.Johnson BR. Considerations in the selection of a root-end filling material. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:398–404. doi: 10.1016/s1079-2104(99)70237-4. [DOI] [PubMed] [Google Scholar]
- 14.Gutmann JL, Pitt Ford TR. Management of the resected root end: a clinical review. Int Endod J. 1993;26:273–83. doi: 10.1111/j.1365-2591.1993.tb00572.x. [DOI] [PubMed] [Google Scholar]
- 15.Reyes-Carmona JF, Felippe MS, Felippe WT. A phosphate-buffered saline intracanal dressing improves the biomineralization ability of mineral trioxide aggregate apical plugs. J Endod. 2010;36:1648–52. doi: 10.1016/j.joen.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Heling I, Chandler NP. The antimicrobial effect within dentinal tubules of four root canal sealers. J Endod. 1996;22:257–9. doi: 10.1016/s0099-2399(06)80144-5. [DOI] [PubMed] [Google Scholar]
- 17.Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review—part III: clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36:400–13. doi: 10.1016/j.joen.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Torabinejad M, Hong CU, Lee SJ, Monsef M, Pitt Ford TR. Investigation of mineral trioxide aggregate for root-end filling in dogs. J Endod. 1995;21:603–8. doi: 10.1016/S0099-2399(06)81112-X. [DOI] [PubMed] [Google Scholar]
- 19.Bonson S, Jeansonne BG, Lallier TE. Root-end filling materials alter fibroblast differentiation. J Dent Res. 2004;83:408–13. doi: 10.1177/154405910408300511. [DOI] [PubMed] [Google Scholar]
- 20.Torabinejad M, Pitt Ford TR, McKendry DJ, Abedi HR, Miller DA, Kariyawasam SP. Histologic assessment of mineral trioxide aggregate as a root-end filling in monkeys. J Endod. 1997;23:225–8. doi: 10.1016/S0099-2399(97)80051-9. [DOI] [PubMed] [Google Scholar]
- 21.Asrari M, Lobner D. In vitro neurotoxic evaluation of root-end-filling materials. J Endod. 2003;29:743–6. doi: 10.1097/00004770-200311000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Balto HA. Attachment and morphological behavior of human periodontal ligament fibroblasts to mineral trioxide aggregate: a scanning electron microscope study. J Endod. 2004;30:25–9. doi: 10.1097/00004770-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Sarkar NK, Caicedo R, Ritwik P, Moiseyeva R, Kawashima I. Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod. 2005;31:97–100. doi: 10.1097/01.don.0000133155.04468.41. [DOI] [PubMed] [Google Scholar]
- 24.Chng HK, Islam I, Yap AU, Tong YW, Koh ET. Properties of a new root-end filling material. J Endod. 2005;31:665–8. doi: 10.1097/01.don.0000157993.89164.be. [DOI] [PubMed] [Google Scholar]
- 25.Kogan P, He J, Glickman GN, Watanabe I. The effects of various additives on setting properties of MTA. J Endod. 2006;32:569–72. doi: 10.1016/j.joen.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Torabinejad M, Hong CU, McDonald F, Pitt Ford TR. Physical and chemical properties of a new root-end filling material. J Endod. 1995;21:349–53. doi: 10.1016/S0099-2399(06)80967-2. [DOI] [PubMed] [Google Scholar]
- 27.Porter ML, Berto A, Primus CM, Watanabe I. Physical and chemical properties of new-generation endodontic materials. J Endod. 2010;36:524–8. doi: 10.1016/j.joen.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Bortoluzzi EA, Broon NJ, Bramante CM, Garcia RB, de Moraes IG, Bernardineli N. Sealing ability of MTA and radiopaque Portland cement with or without calcium chloride for root-end filling. J Endod. 2006;32:897–900. doi: 10.1016/j.joen.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 29.von Arx T, Hanni S, Storgard SS. Clincial results with two different methods of root-end preparation and filling in apical surgery: mineral trioxide aggregate and adhesive resin composite. J Endod. 2010;36:1122–9. doi: 10.1016/j.joen.2010.03.040. [DOI] [PubMed] [Google Scholar]
- 30.Kokkas AB, Boutsioukis A, Vassiliadis LP, Stavrianos CK. The influence of the smear layer on dentinal tubule penetration depth by three different root canal sealers: an in vitro study. J Endod. 2004;30:100–2. doi: 10.1097/00004770-200402000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Kouvas V, Liolios E, Vassiliadis L, Parissis-Messimeris S, Boutsioukis A. Influence of smear layer on depth of penetration of three endodontic sealers: an SEM study. Endod Dent Traumatol. 1998;14:191–5. doi: 10.1111/j.1600-9657.1998.tb00836.x. [DOI] [PubMed] [Google Scholar]
- 32.Dreger LA, Felippe WT, Reyes-Carmona JF, Felippe GS, Bortoluzzi EA, Felippe MC. Mineral trioxide aggregate and Portland cement promote biomineralization in vivo. J Endod. 2012;38:324–9. doi: 10.1016/j.joen.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Garberoglio R, Brannstrom M. Scanning electron microscopic investigation of human dentinal tubules. Arch Oral Biol. 1976;21:355–62. doi: 10.1016/s0003-9969(76)80003-9. [DOI] [PubMed] [Google Scholar]
- 34.Ten Cate AR. Oral histology: development, structure, and function. 5. St Louis, MO: Mosby; 1998. [Google Scholar]
- 35.Komabayashi T, Spangberg LS. Comparative analysis of the particle size and shape of commercially available mineral trioxide aggregates and Portland cement: a study with a flow particle image analyzer. J Endod. 2008;34:94–8. doi: 10.1016/j.joen.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 36.Ma J, Shen Y, Stojicic S, Haapasalo M. Biocompatibility of two novel root repair materials. J Endod. 2011;37:793–8. doi: 10.1016/j.joen.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 37.Holland R, de Souza V, Nery MJ, Otoboni Filho JA, Bernabe PF, Dezan Junior E. Reaction of rat connective tissue to implanted dentin tubes filled with mineral trioxide aggregate or calcium hydroxide. J Endod. 1999;25:161–6. doi: 10.1016/s0099-2399(99)80134-4. [DOI] [PubMed] [Google Scholar]
- 38.Chohayeb AA, Chow LC, Tsaknis PJ. Evaluation of calcium phosphate as a root canal sealer-filler material. J Endod. 1987;13:384–7. doi: 10.1016/S0099-2399(87)80198-X. [DOI] [PubMed] [Google Scholar]
- 39.Sugawara A, Fujikawa K, Kusama K, et al. Histopathologic reaction of a calcium phosphate cement for alveolar ridge augmentation. J Biomed Mater Res. 2002;61:47–52. doi: 10.1002/jbm.10010. [DOI] [PubMed] [Google Scholar]
- 40.Mangin C, Yesilsoy C, Nissan R, Stevens R. The comparative sealing ability of hydroxyapatite cement, mineral trioxide aggregate, and super ethoxybenzoic acid as root-end filling materials. J Endod. 2003;29:261–4. doi: 10.1097/00004770-200304000-00008. [DOI] [PubMed] [Google Scholar]


