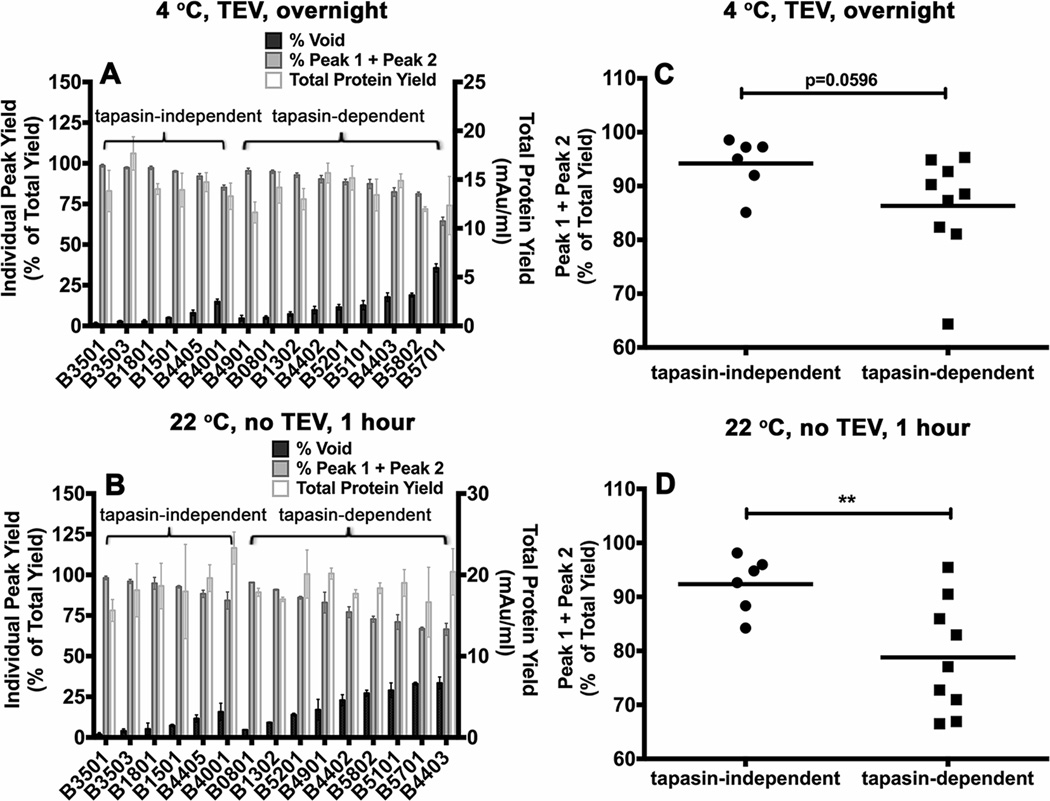
Figure 4. Variable levels of aggregation of soluble empty HLA-B molecules following refolding.
Soluble forms of the indicated MHC class I heavy chains were refolded with β2m A) overnight at 4 °C with TEV or B) at room temperature (22 ° C) for 1 hour without TEV. Following refolding, soluble proteins were analyzed by gel filtration chromatography. Percentages of fractions corresponding to the void volume and Peak1 + Peak 2 (peptide binding competent fractions) of different allotypes are shown (left Y axis) and total protein yields (right Y axis) are also shown. Data represent averaged values from three independent refolding reactions with two different inclusion body preparations for all allotypes except HLA-B*0801 and HLA-B*1302, which represent averaged values of two independent refolding reactions with a single inclusion body preparation. C and D) Percentages of Peak 1+ Peak 2 fractions, derived from data in A and B, were compared for the tapasin-independent and tapasin-dependent allotypes. Differences between two groups are significant when refolding is conducted under the more stringent conditions (1 hour at 22 °C). Statistical analyses were performed using an unpaired t-test. ** Indicates that the P value was < 0. 01.