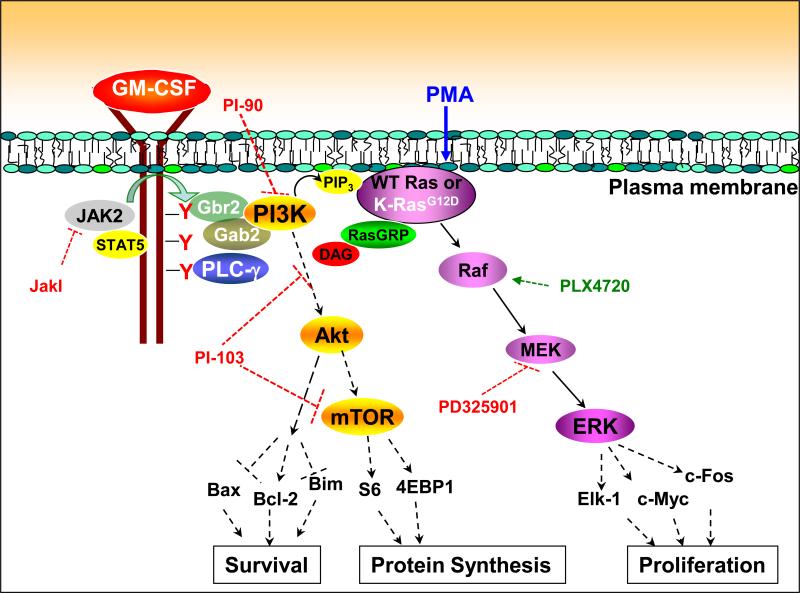
Fig. 6. Proposed mechanism of cytokine-induced Ras and ERK activation in hematopoietic cells expressing WT and oncogenic K-Ras.
In response the binding of GM-CSF to its receptor, JAK2 trans-phosphorylates multiple tyrosine residues on the β common chain of the GM-CSF receptor, creating docking sites for adaptor proteins and signaling molecules. PLC-γ1 and PLC-γ2 are recruited to the receptor complex and becomes activated, generating DAG. The p85 regulatory subunit of PI3K binds to the receptor through adaptor proteins, which results in PI3K activation and PIP3 production. Second messengers generated by PI3K and PLC-γ stimulate nucleotide exchange on Ras, likely by both localizing Ras to the plasma membrane and activating RasGRPs. The DAG mimetic PMA activates Ras and ERK independently of PLC-γ and PI3K. This scheme does not directly address the interaction between PLC-γ and PI3K; however, multiple lines of evidence suggested that PLC-γ is upstream of PI3K. The levels at which the different inhibitors used in this study exerted their effects are indicated by dashed red bars. Raf activation by PLX4720 is indicated as a dashed green arrow. Studies using many of these inhibitors support a similar role for PLC-γ and PI3K in linking the activated receptor tyrosine kinase c-Kit to Ras and ERK signaling.