Abstract
We have compared the effects of the complement membrane attack complex (MAC), nystatin, and melittin on the envelope of murine leukemia viruses to determine if channel formation alone is sufficient to cause membranolysis. Nystatin is a channel former and mellitin is not, although both are hemolytic. Whereas MAC and melittin disintegrated the viral membrane, nystatin had no effect on morphology, integrity, and infectivity of the virus. Incorporation of the antibiotic into the viral membranes was demonstrated by measurements of the characteristic fluorescence of nystatin in membranes and the dose-dependent increase in viral density after uptake of the antibiotic. The density of nystatin was measured to be 1.26-1.27 g/cm3. Proof for the formation of functional nystatin channels was obtained by light scattering measurements. Exposure of untreated virus to hypotonic conditions increased viral light scattering because of osmotic swelling but otherwise had no effect on the integrity of the virus. Nystatin channel formation abolished the light scattering change, showing that the antibiotic had impaired the viral permeability barrier. We interpret these results to indicate that virolysis by MAC is not caused by channel formation and, conversely, in the absence of colloid-osmotic effects, channel formation by itself is not sufficient to disassemble a viral membrane.
Full text
PDF
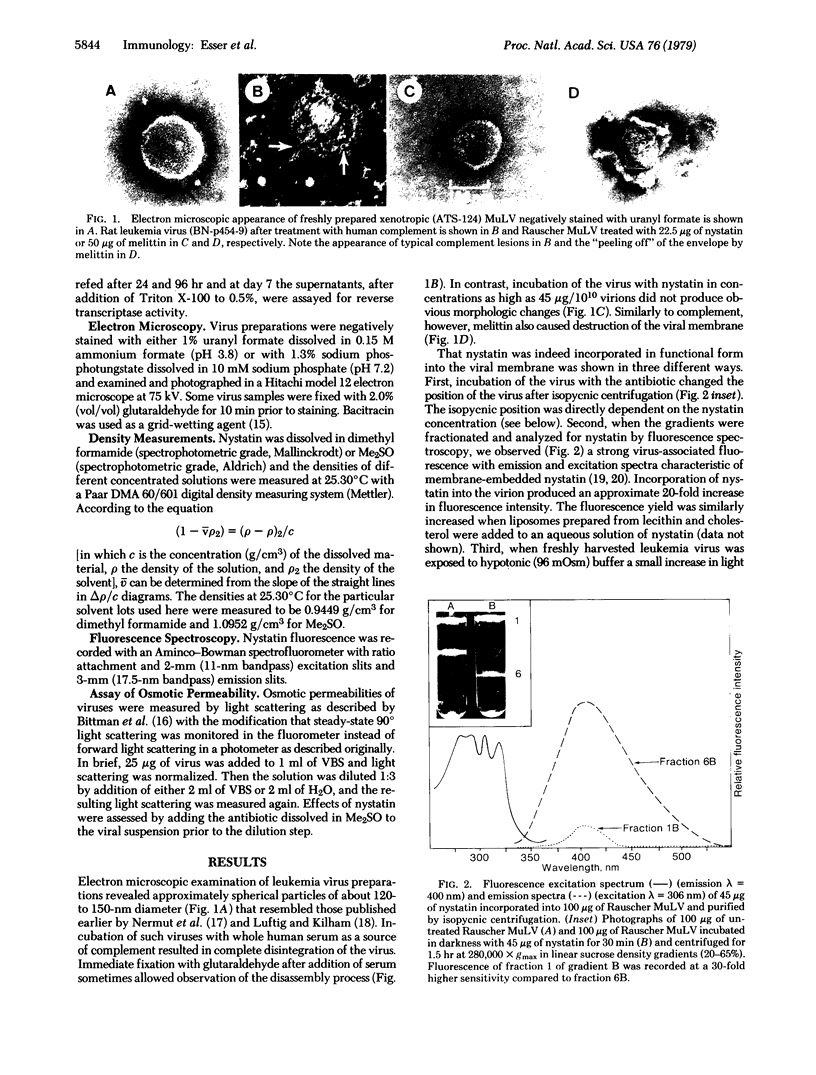
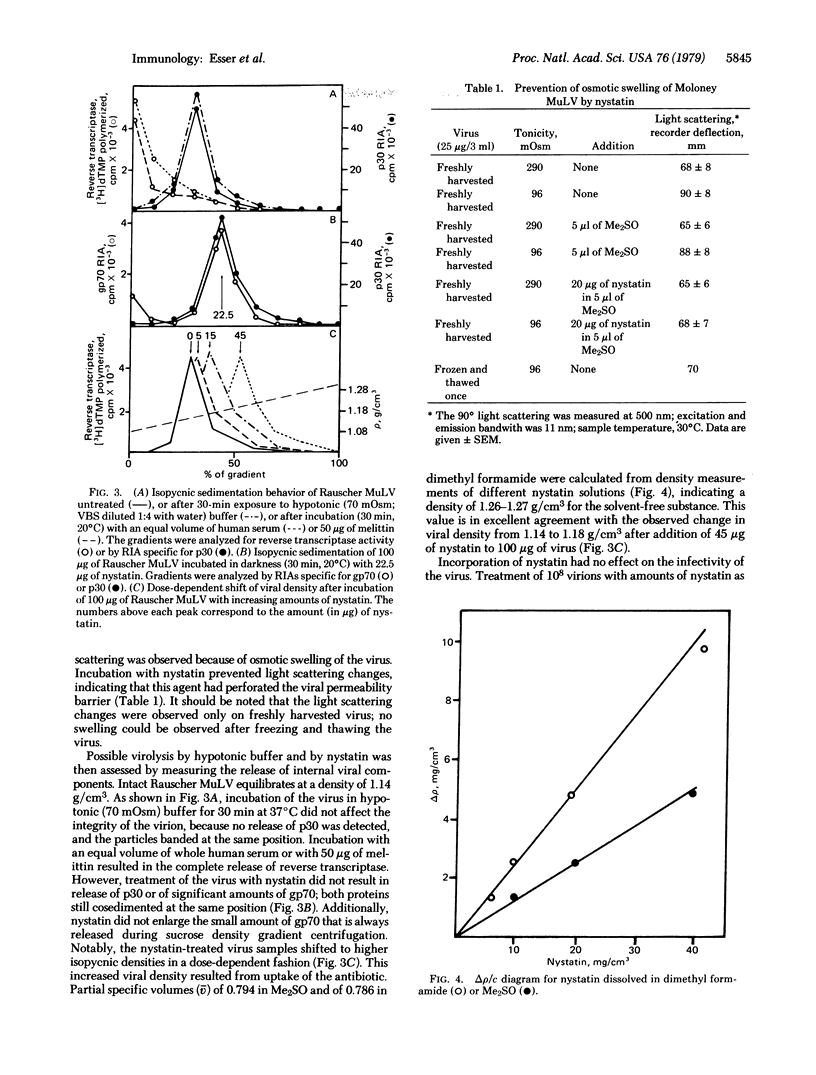


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreoli T. E. On the anatomy of amphotericin B-cholesterol pores in lipid bilayer membranes. Kidney Int. 1973 Nov;4(5):337–345. doi: 10.1038/ki.1973.126. [DOI] [PubMed] [Google Scholar]
- Bartholomew R. M., Esser A. F., Müller-Eberhard H. J. Lysis of oncornaviruses by human serum. Isolation of the viral complement (C1) receptor and identification as p15E. J Exp Med. 1978 Mar 1;147(3):844–853. doi: 10.1084/jem.147.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittman R., Fischkoff S. A. Fluorescence studies of the binding of the polyene antibiotics filipin 3, amphotericin B, nystatin, and lagosin to cholesterol. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3795–3799. doi: 10.1073/pnas.69.12.3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittman R., Majuk Z., Honig D. S., Compans R. W., Lenard J. Permeability properties of the membrane of vesicular stomatitis virions. Biochim Biophys Acta. 1976 Apr 16;433(1):63–74. doi: 10.1016/0005-2736(76)90178-4. [DOI] [PubMed] [Google Scholar]
- Bittman R. Sterol-polyene antibiotic complexation: probe of membrane structure. Lipids. 1978 Oct;13(10):686–691. doi: 10.1007/BF02533746. [DOI] [PubMed] [Google Scholar]
- Cass A., Finkelstein A., Krespi V. The ion permeability induced in thin lipid membranes by the polyene antibiotics nystatin and amphotericin B. J Gen Physiol. 1970 Jul;56(1):100–124. doi: 10.1085/jgp.56.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper N. R., Jensen F. C., Welsh R. M., Jr, Oldstone M. B. Lysis of RNA tumor viruses by human serum: direct antibody-independent triggering of the classical complement pathway. J Exp Med. 1976 Oct 1;144(4):970–984. doi: 10.1084/jem.144.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson C. R., Drake A. F., Helliwell J., Hider R. C. The interaction of bee melittin with lipid bilayer membranes. Biochim Biophys Acta. 1978 Jun 16;510(1):75–86. doi: 10.1016/0005-2736(78)90131-1. [DOI] [PubMed] [Google Scholar]
- Decad G. M., Nikaido H. Outer membrane of gram-negative bacteria. XII. Molecular-sieving function of cell wall. J Bacteriol. 1976 Oct;128(1):325–336. doi: 10.1128/jb.128.1.325-336.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufourcq J., Faucon J. F. Intrinsic fluorescence study of lipid-protein interactions in membrane models. Binding of melittin, an amphipathic peptide, to phospholipid vesicles. Biochim Biophys Acta. 1977 May 16;467(1):1–11. doi: 10.1016/0005-2736(77)90236-x. [DOI] [PubMed] [Google Scholar]
- Ermishkin L. N., Kasumov K. M., Potseluyev V. M. Properties of amphotericin B channels in a lipid bilayer. Biochim Biophys Acta. 1977 Nov 1;470(3):357–367. doi: 10.1016/0005-2736(77)90127-4. [DOI] [PubMed] [Google Scholar]
- Esser A. F., Kolb W. P., Podack E. R., Müller-Eberhard H. J. Molecular reorganization of lipid bilayers by complement: a possible mechanism for membranolysis. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1410–1414. doi: 10.1073/pnas.76.3.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby J. A., Götze O., Müller-Eberhard H. J., Kinsky S. C. Release of trapped marker from liposomes by the action of purified complement components. Proc Natl Acad Sci U S A. 1969 Sep;64(1):290–295. doi: 10.1073/pnas.64.1.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izui S., McConahey P. J., Theofilopoulos A. N., Dixon F. J. Association of circulating retroviral gp70-anti-gp70 immune complexes with murine systemic lupus erythematosus. J Exp Med. 1979 May 1;149(5):1099–1116. doi: 10.1084/jem.149.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINSKY S. C. COMPARATIVE RESPONSES OF MAMMALIAN ERYTHROCYTES AND MICROBIAL PROTOPLASTS TO POLYENE ANTIBIOTICS AND VITAMIN A. Arch Biochem Biophys. 1963 Aug;102:180–188. doi: 10.1016/0003-9861(63)90169-3. [DOI] [PubMed] [Google Scholar]
- Leberman R. Use of uranyl formate as a negative stain. J Mol Biol. 1965 Sep;13(2):606–606. doi: 10.1016/s0022-2836(65)80124-3. [DOI] [PubMed] [Google Scholar]
- Luftig R. B., Kilham S. S. An electron microscope study of Rauscher leukemia virus. Virology. 1971 Nov;46(2):277–297. doi: 10.1016/0042-6822(71)90030-4. [DOI] [PubMed] [Google Scholar]
- Majuk Z., Bittman R., Landsberger F. R., Compans R. W. Effects of filipin on the structure and biological activity of enveloped viruses. J Virol. 1977 Dec;24(3):883–892. doi: 10.1128/jvi.24.3.883-892.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt H., Gilden R. V., Oroszlan S. Envelope glycoproteins of Rauscher murine leukemia virus: isolation and chemical characterization. Biochemistry. 1977 Feb 22;16(4):710–717. doi: 10.1021/bi00623a024. [DOI] [PubMed] [Google Scholar]
- Mayer M. M. Complement, past and present. Harvey Lect. 1978;72:139–193. [PubMed] [Google Scholar]
- Mayer M. M. Mechanism of cytolysis by complement. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2954–2958. doi: 10.1073/pnas.69.10.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels D. W., Abramovitz A. S., Hammer C. H., Mayer M. M. Increased ion permeability of planar lipid bilayer membranes after treatment with the C5b-9 cytolytic attack mechanism of complement. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2852–2856. doi: 10.1073/pnas.73.8.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollay C. Effect of melitin and melittin fragments on the thermotropic phase transition of dipalmitoyllecithin and on the amount of lipid-bound water. FEBS Lett. 1976 Apr 15;64(1):65–68. doi: 10.1016/0014-5793(76)80250-5. [DOI] [PubMed] [Google Scholar]
- Nermut M. V., Frank H., Schäfer W. Properties of mouse leukemia viruses. 3. Electron microscopic appearance as revealed after conventional preparation techniques as well as freeze-drying and freeze-etching. Virology. 1972 Aug;49(2):345–358. doi: 10.1016/0042-6822(72)90487-4. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Gilden R. V. Immune virolysis: effect of antibody and complement on C-type RNA virus. Science. 1970 Jun 19;168(3938):1478–1480. doi: 10.1126/science.168.3938.1478. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Biesecker G., Müller-Eberhard H. J. Membrane attack complex of complement: generation of high-affinity phospholipid binding sites by fusion of five hydrophilic plasma proteins. Proc Natl Acad Sci U S A. 1979 Feb;76(2):897–901. doi: 10.1073/pnas.76.2.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R. D., Morrison D. C., Podack E. R., Müller-Eberhard H. J. Bactericidal activity of the alternative complement pathway generated from 11 isolated plasma proteins. J Exp Med. 1979 Apr 1;149(4):870–882. doi: 10.1084/jem.149.4.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder F., Holland J. F., Bieber L. L. Fluorometric investigations of the interaction of polyene antibiotics with sterols. Biochemistry. 1972 Aug 1;11(16):3105–3111. doi: 10.1021/bi00766a026. [DOI] [PubMed] [Google Scholar]
- Sessa G., Freer J. H., Colacicco G., Weissmann G. Interaction of alytic polypeptide, melittin, with lipid membrane systems. J Biol Chem. 1969 Jul 10;244(13):3575–3582. [PubMed] [Google Scholar]
- Verma S. P., Wallach D. F., Smith I. C. The action of melittin on phosphatide multibilayers as studied by infrared dichroism and spin labeling. A model approach to lipid-protein interactions. Biochim Biophys Acta. 1974 Apr 12;345(1):129–140. doi: 10.1016/0005-2736(74)90252-1. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Jr, Cooper N. R., Jensen F. C., Oldstone M. B. Human serum lyses RNA tumour viruses. Nature. 1975 Oct 16;257(5527):612–614. doi: 10.1038/257612a0. [DOI] [PubMed] [Google Scholar]
- Wobschall D., McKeon C. Step conductance increases in bilayer membranes induced by antibody-antigen-complement action. Biochim Biophys Acta. 1975 Dec 1;413(2):317–321. doi: 10.1016/0005-2736(75)90117-0. [DOI] [PubMed] [Google Scholar]